Page 69 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 69
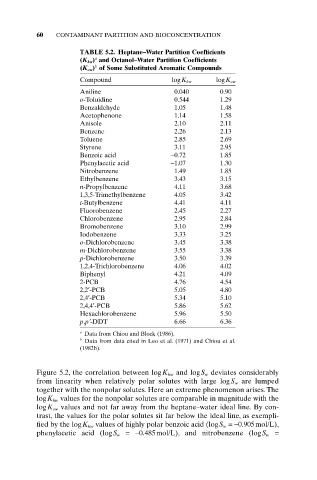
60 CONTAMINANT PARTITION AND BIOCONCENTRATION
TABLE 5.2. Heptane–Water Partition Coefficients
a
(K hw) and Octanol–Water Partition Coefficients
b
(K ow) of Some Substituted Aromatic Compounds
Compound logK hw logK ow
Aniline 0.040 0.90
o-Toluidine 0.544 1.29
Benzaldehyde 1.05 1.48
Acetophenone 1.14 1.58
Anisole 2.10 2.11
Benzene 2.26 2.13
Toluene 2.85 2.69
Styrene 3.11 2.95
Benzoic acid -0.72 1.85
Phenylacetic acid -1.07 1.30
Nitrobenzene 1.49 1.85
Ethylbenzene 3.43 3.15
n-Propylbenzene 4.11 3.68
1,3,5-Trimethylbenzene 4.05 3.42
t-Butylbenzene 4.41 4.11
Fluorobenzene 2.45 2.27
Chlorobenzene 2.95 2.84
Bromobenzene 3.10 2.99
Iodobenzene 3.33 3.25
o-Dichlorobenzene 3.45 3.38
m-Dichlorobenzene 3.55 3.38
p-Dichlorobenzene 3.50 3.39
1,2,4-Trichlorobenzene 4.06 4.02
Biphenyl 4.21 4.09
2-PCB 4.76 4.54
2,2¢-PCB 5.05 4.80
2,4¢-PCB 5.34 5.10
2,4,4¢-PCB 5.86 5.62
Hexachlorobenzene 5.96 5.50
p,p¢-DDT 6.66 6.36
a Data from Chiou and Block (1986).
b Data from data cited in Leo et al. (1971) and Chiou et al.
(1982b).
Figure 5.2, the correlation between logK hw and logS w deviates considerably
from linearity when relatively polar solutes with large logS w are lumped
together with the nonpolar solutes. Here an extreme phenomenon arises. The
logK hw values for the nonpolar solutes are comparable in magnitude with the
logK ow values and not far away from the heptane–water ideal line. By con-
trast, the values for the polar solutes sit far below the ideal line, as exempli-
fied by the logK hw values of highly polar benzoic acid (logS w =-0.905mol/L),
phenylacetic acid (logS w =-0.485mol/L), and nitrobenzene (logS w =