Page 282 - Petroleum and Gas Field Processing
P. 282
11.5.1 Amine Processes
The most widely used for sweetening of natural gas are aqueous solutions
of alkanolamines. They are generally used for bulk removal of CO 2 and
H 2 S. The properties of several amines are shown in Table 2. The low
operating cost and flexibility of tailoring solvent composition to suit gas
compositions make this process one of most commonly selected. A liquid
physical solvent can be added to the amine to improve selectivity.
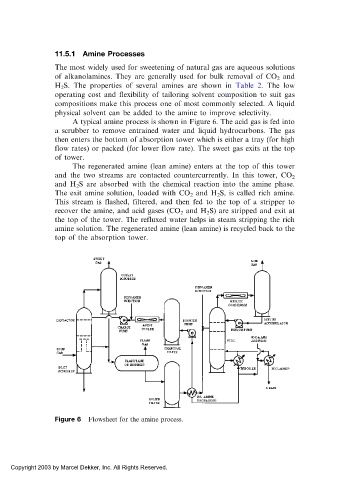
A typical amine process is shown in Figure 6. The acid gas is fed into
a scrubber to remove entrained water and liquid hydrocarbons. The gas
then enters the bottom of absorption tower which is either a tray (for high
flow rates) or packed (for lower flow rate). The sweet gas exits at the top
of tower.
The regenerated amine (lean amine) enters at the top of this tower
and the two streams are contacted countercurrently. In this tower, CO 2
and H 2 S are absorbed with the chemical reaction into the amine phase.
The exit amine solution, loaded with CO 2 and H 2 S, is called rich amine.
This stream is flashed, filtered, and then fed to the top of a stripper to
recover the amine, and acid gases (CO 2 and H 2 S) are stripped and exit at
the top of the tower. The refluxed water helps in steam stripping the rich
amine solution. The regenerated amine (lean amine) is recycled back to the
top of the absorption tower.
Figure 6 Flowsheet for the amine process.
Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved.