Page 279 - Petroleum and Gas Field Processing
P. 279
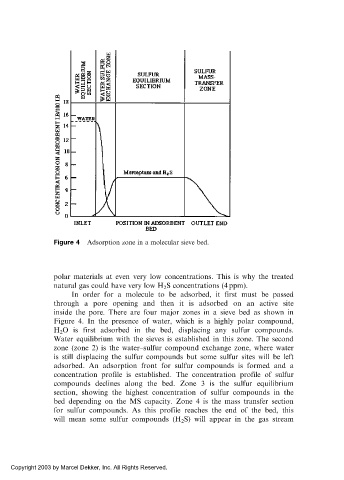
Figure 4 Adsorption zone in a molecular sieve bed.
polar materials at even very low concentrations. This is why the treated
natural gas could have very low H 2 S concentrations (4 ppm).
In order for a molecule to be adsorbed, it first must be passed
through a pore opening and then it is adsorbed on an active site
inside the pore. There are four major zones in a sieve bed as shown in
Figure 4. In the presence of water, which is a highly polar compound,
H 2 O is first adsorbed in the bed, displacing any sulfur compounds.
Water equilibrium with the sieves is established in this zone. The second
zone (zone 2) is the water–sulfur compound exchange zone, where water
is still displacing the sulfur compounds but some sulfur sites will be left
adsorbed. An adsorption front for sulfur compounds is formed and a
concentration profile is established. The concentration profile of sulfur
compounds declines along the bed. Zone 3 is the sulfur equilibrium
section, showing the highest concentration of sulfur compounds in the
bed depending on the MS capacity. Zone 4 is the mass transfer section
for sulfur compounds. As this profile reaches the end of the bed, this
will mean some sulfur compounds (H 2 S) will appear in the gas stream
Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved.