Page 276 - Petroleum and Gas Field Processing
P. 276
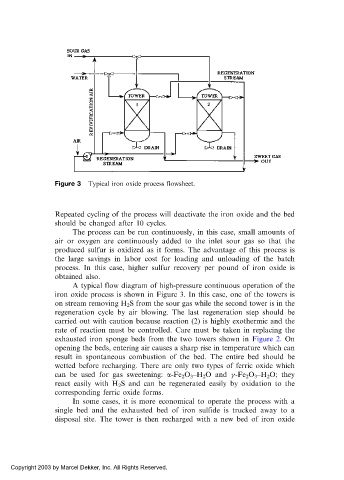
Figure 3 Typical iron oxide process flowsheet.
Repeated cycling of the process will deactivate the iron oxide and the bed
should be changed after 10 cycles.
The process can be run continuously, in this case, small amounts of
air or oxygen are continuously added to the inlet sour gas so that the
produced sulfur is oxidized as it forms. The advantage of this process is
the large savings in labor cost for loading and unloading of the batch
process. In this case, higher sulfur recovery per pound of iron oxide is
obtained also.
A typical flow diagram of high-pressure continuous operation of the
iron oxide process is shown in Figure 3. In this case, one of the towers is
on stream removing H 2 S from the sour gas while the second tower is in the
regeneration cycle by air blowing. The last regeneration step should be
carried out with caution because reaction (2) is highly exothermic and the
rate of reaction must be controlled. Care must be taken in replacing the
exhausted iron sponge beds from the two towers shown in Figure 2. On
opening the beds, entering air causes a sharp rise in temperature which can
result in spontaneous combustion of the bed. The entire bed should be
wetted before recharging. There are only two types of ferric oxide which
can be used for gas sweetening: a-Fe 2 O 3 –H 2 O and
-Fe 2 O 3 –H 2 O; they
react easily with H 2 S and can be regenerated easily by oxidation to the
corresponding ferric oxide forms.
In some cases, it is more economical to operate the process with a
single bed and the exhausted bed of iron sulfide is trucked away to a
disposal site. The tower is then recharged with a new bed of iron oxide
Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved.