Page 316 - Petroleum and Gas Field Processing
P. 316
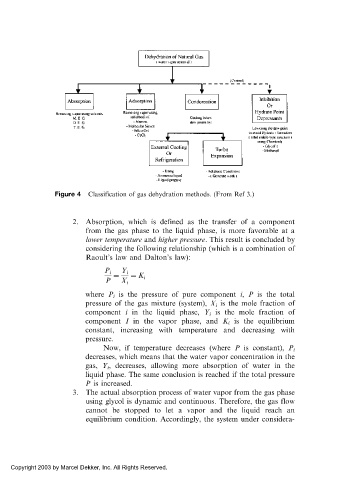
Figure 4 Classification of gas dehydration methods. (From Ref 3.)
2. Absorption, which is defined as the transfer of a component
from the gas phase to the liquid phase, is more favorable at a
lower temperature and higher pressure. This result is concluded by
considering the following relationship (which is a combination of
Raoult’s law and Dalton’s law):
P i Y i
¼ ¼ K i
P X i
where P i is the pressure of pure component i, P is the total
pressure of the gas mixture (system), X i is the mole fraction of
component i in the liquid phase, Y i is the mole fraction of
component I in the vapor phase, and K i is the equilibrium
constant, increasing with temperature and decreasing with
pressure.
Now, if temperature decreases (where P is constant), P i
decreases, which means that the water vapor concentration in the
gas, Y i , decreases, allowing more absorption of water in the
liquid phase. The same conclusion is reached if the total pressure
P is increased.
3. The actual absorption process of water vapor from the gas phase
using glycol is dynamic and continuous. Therefore, the gas flow
cannot be stopped to let a vapor and the liquid reach an
equilibrium condition. Accordingly, the system under considera-
Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved.