Page 154 - Photonics Essentials an introduction with experiments
P. 154
Lasers
148 Photonic Devices
N 2
Stimulated
Emission
N 1
(a)
N 1
Stimulated
Absorption
N 1
(b)
N 1
Spontaneous
Emission
N 1
(c)
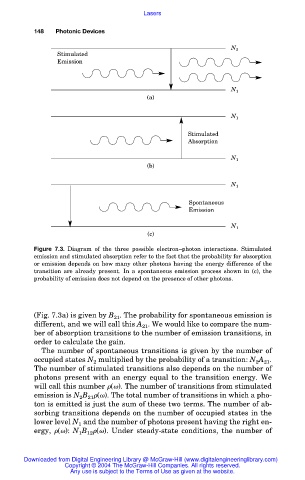
Figure 7.3. Diagram of the three possible electron–photon interactions. Stimulated
emission and stimulated absorption refer to the fact that the probability for absorption
or emission depends on how many other photons having the energy difference of the
transition are already present. In a spontaneous emission process shown in (c), the
probability of emission does not depend on the presence of other photons.
(Fig. 7.3a) is given by B 21 . The probability for spontaneous emission is
different, and we will call this A 21 . We would like to compare the num-
ber of absorption transitions to the number of emission transitions, in
order to calculate the gain.
The number of spontaneous transitions is given by the number of
occupied states N multiplied by the probability of a transition: N 2 A 21 .
2
The number of stimulated transitions also depends on the number of
photons present with an energy equal to the transition energy. We
will call this number ( ). The number of transitions from stimulated
emission is N 2 B 21 ( ). The total number of transitions in which a pho-
ton is emitted is just the sum of these two terms. The number of ab-
sorbing transitions depends on the number of occupied states in the
lower level N and the number of photons present having the right en-
1
ergy, ( ): N 1 B 12 ( ). Under steady-state conditions, the number of
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.