Page 189 - Physical chemistry understanding our chemical world
P. 189
156 REACTION SPONTANEITY AND THE DIRECTION OF THERMODYNAMIC CHANGE
where p is conventional pressure and γ is the fugacity coefficient representing the
deviation from ideality. Values of γ can be measured or calculated.
We employ both concepts to compensate for gas non-ideality.
4.5 Thermodynamics and the extent
of reaction
Why is a ‘weak’ acid weak?
Incomplete reactions and extent of reaction
As chemists, we should perhaps re-cast the question ‘Why is a ‘weak’ acid weak?’ by
asking ‘How does the change in Gibbs function relate to the proportion of reactants
that convert during a reaction to form products?’
An acid is defined as a proton donor within the Lowry–Brønsted theory (see
Chapter 6). Molecules of acid ionize in aqueous solution to form an anion and a
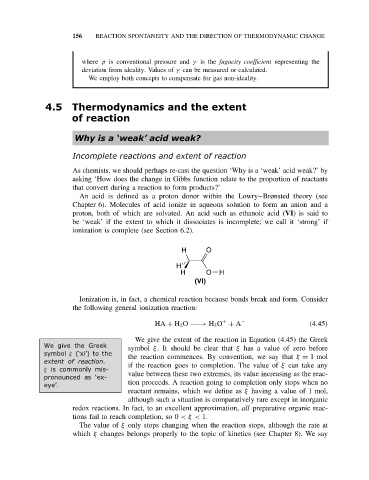
proton, both of which are solvated. An acid such as ethanoic acid (VI)issaidto
be ‘weak’ if the extent to which it dissociates is incomplete; we call it ‘strong’ if
ionization is complete (see Section 6.2).
H O
H
H O H
(VI)
Ionization is, in fact, a chemical reaction because bonds break and form. Consider
the following general ionization reaction:
+
HA + H 2 O −−→ H 3 O + A − (4.45)
We give the extent of the reaction in Equation (4.45) the Greek
We give the Greek symbol ξ. It should be clear that ξ has a value of zero before
symbol ξ (‘xi’) to the the reaction commences. By convention, we say that ξ = 1mol
extent of reaction. if the reaction goes to completion. The value of ξ can take any
ξ is commonly mis-
pronounced as ‘ex- value between these two extremes, its value increasing as the reac-
tion proceeds. A reaction going to completion only stops when no
eye’.
reactant remains, which we define as ξ having a value of 1 mol,
although such a situation is comparatively rare except in inorganic
redox reactions. In fact, to an excellent approximation, all preparative organic reac-
tions fail to reach completion, so 0 <ξ < 1.
The value of ξ only stops changing when the reaction stops, although the rate at
which ξ changes belongs properly to the topic of kinetics (see Chapter 8). We say