Page 190 - Physical chemistry understanding our chemical world
P. 190
THERMODYNAMICS AND THE EXTENT OF REACTION 157
it reaches its position of equilibrium, for which the value of ξ has
We assume such an
its equilibrium value ξ (eq) . We propose perhaps the simplest of the
equilibrium is fully
many possible definitions of equilibrium: ‘after an initial period of
reversible in the sense
reaction, no further net changes in reaction composition occur’. of being dynamic –the
So when we say that a carboxylic acid is weak, we mean that rate at which products
ξ (eq) is small. Note how, by saying that ξ (eq) is small at equilibrium, form is equal and oppo-
we effectively imply that the extent of ionization is small because site to the rate at which
−
+
[H 3 O ] and [A ] are both small. reactants regenerate
But we need to be careful when talking about the magnitudes viaabackreaction.
of ξ. Consider the case of sodium ethanoate dissolved in dilute
mineral acid: the reaction occurring is, in fact, the reverse of that
in Equation (4.45), with a proton and carboxylate anion associat-
Reminder: the energy
ing to form undissociated acid. In this case, ξ = 1 mol before the
released during reac-
reaction occurs, and its value decreases as the reaction proceeds.
tion originates from
In other words, we need to define our reaction before we can speak
the making and break-
knowledgeably about it. We can now rewrite our question, asking ing of bonds, and the
‘Why is ξ 1 for a weak acid?’ rearrangement of sol-
O
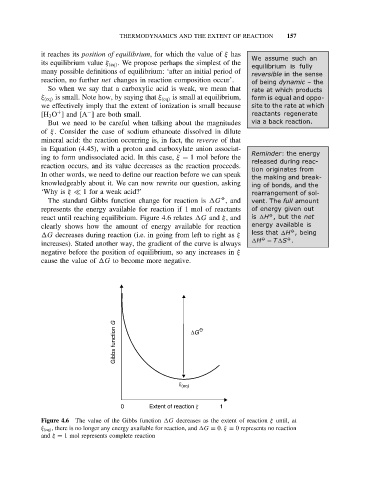
The standard Gibbs function change for reaction is G , and vent. The full amount
represents the energy available for reaction if 1 mol of reactants of energy given out
O
react until reaching equilibrium. Figure 4.6 relates G and ξ, and is H ,but the net
clearly shows how the amount of energy available for reaction energy available is
O
less that H ,being
G decreases during reaction (i.e. in going from left to right as ξ
O
H − T S .
O
increases). Stated another way, the gradient of the curve is always
negative before the position of equilibrium, so any increases in ξ
cause the value of G to become more negative.
Gibbs function G ∆G O
x (eq)
0 Extent of reaction x 1
Figure 4.6 The value of the Gibbs function G decreases as the extent of reaction ξ until, at
ξ (eq) , there is no longer any energy available for reaction, and G = 0. ξ = 0 represents no reaction
and ξ = 1 mol represents complete reaction