Page 244 - Physical chemistry understanding our chemical world
P. 244
PHASE EQUILIBRIA INVOLVING TWO-COMPONENT SYSTEMS: PARTITION 211
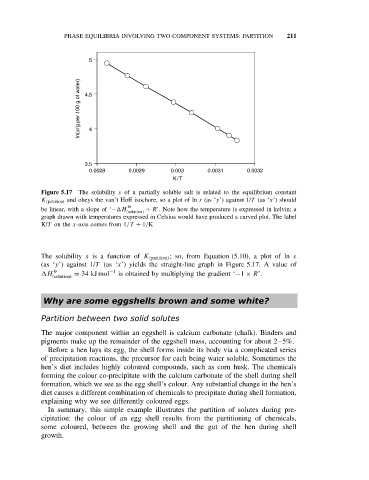
5
ln(s/g per 100 g of water) 4.5 4
3.5
0.0028 0.0029 0.003 0.0031 0.0032
K/T
Figure 5.17 The solubility s of a partially soluble salt is related to the equilibrium constant
K (partition) and obeys the van’t Hoff isochore, so a plot of ln s (as ‘y’) against 1/T (as ‘x’) should
be linear, with a slope of ‘− H O ÷ R’. Note how the temperature is expressed in kelvin; a
(solution)
graph drawn with temperatures expressed in Celsius would have produced a curved plot. The label
K/T on the x-axis comes from 1/T ÷ 1/K
The solubility s is a function of K (partition) ; so, from Equation (5.10), a plot of ln s
(as ‘y’) against 1/T (as ‘x’) yields the straight-line graph in Figure 5.17. A value of
H O = 34 kJ mol −1 is obtained by multiplying the gradient ‘−1 × R’.
(solution)
Why are some eggshells brown and some white?
Partition between two solid solutes
The major component within an eggshell is calcium carbonate (chalk). Binders and
pigments make up the remainder of the eggshell mass, accounting for about 2–5%.
Before a hen lays its egg, the shell forms inside its body via a complicated series
of precipitation reactions, the precursor for each being water soluble. Sometimes the
hen’s diet includes highly coloured compounds, such as corn husk. The chemicals
forming the colour co-precipitate with the calcium carbonate of the shell during shell
formation, which we see as the egg shell’s colour. Any substantial change in the hen’s
diet causes a different combination of chemicals to precipitate during shell formation,
explaining why we see differently coloured eggs.
In summary, this simple example illustrates the partition of solutes during pre-
cipitation: the colour of an egg shell results from the partitioning of chemicals,
some coloured, between the growing shell and the gut of the hen during shell
growth.