Page 249 - Physical chemistry understanding our chemical world
P. 249
216 PHASE EQUILIBRIA
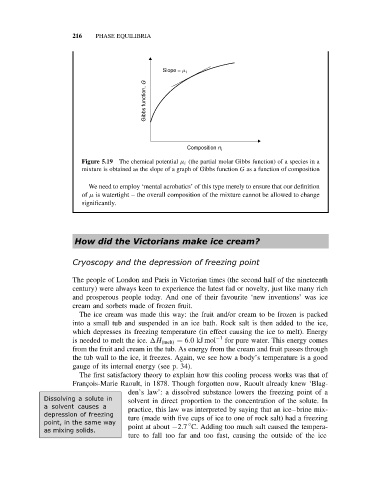
Slope = m i
Gibbs function, G
Composition n i
Figure 5.19 The chemical potential µ i (the partial molar Gibbs function) of a species in a
mixture is obtained as the slope of a graph of Gibbs function G as a function of composition
We need to employ ‘mental acrobatics’ of this type merely to ensure that our definition
of µ is watertight – the overall composition of the mixture cannot be allowed to change
significantly.
How did the Victorians make ice cream?
Cryoscopy and the depression of freezing point
The people of London and Paris in Victorian times (the second half of the nineteenth
century) were always keen to experience the latest fad or novelty, just like many rich
and prosperous people today. And one of their favourite ‘new inventions’ was ice
cream and sorbets made of frozen fruit.
The ice cream was made this way: the fruit and/or cream to be frozen is packed
into a small tub and suspended in an ice bath. Rock salt is then added to the ice,
which depresses its freezing temperature (in effect causing the ice to melt). Energy
is needed to melt the ice. H (melt) = 6.0kJ mol −1 for pure water. This energy comes
from the fruit and cream in the tub. As energy from the cream and fruit passes through
the tub wall to the ice, it freezes. Again, we see how a body’s temperature is a good
gauge of its internal energy (see p. 34).
The first satisfactory theory to explain how this cooling process works was that of
Fran¸cois-Marie Raoult, in 1878. Though forgotten now, Raoult already knew ‘Blag-
den’s law’: a dissolved substance lowers the freezing point of a
Dissolving a solute in solvent in direct proportion to the concentration of the solute. In
a solvent causes a practice, this law was interpreted by saying that an ice–brine mix-
depression of freezing ture (made with five cups of ice to one of rock salt) had a freezing
point, in the same way ◦
as mixing solids. point at about −2.7 C. Adding too much salt caused the tempera-
ture to fall too far and too fast, causing the outside of the ice