Page 250 - Physical chemistry understanding our chemical world
P. 250
PHASE EQUILIBRIA AND COLLIGATIVE PROPERTIES 217
cream to freeze prematurely while the core remained liquid. Adding
The word ‘cryoscopy’
too little salt meant that the ice did not melt, or remained at a tem-
◦
perature close to 0 C, so the cream and fruit juices remained liquid. comes from the Greek
kryos, which literally
This depression of the freezing point occurs in just the same
means ‘frost’.
way as the lower melting point of an impure sample, as discussed
previously. This determination of the depression of the freezing
point is termed crysoscopy.
Why boil vegetables in salted water?
Ebullioscopy and the elevation of boiling point
We often boil vegetables in salted water (the concentration of table salt is usually
−3
in the range 0.01–0.05 mol dm ). The salt makes the food taste nicer, although we
should wash off any excess salt water if we wish to maintain a healthy blood pressure.
But salted water boils at a higher temperature than does pure
water, so the food cooks more quickly. (We saw on p. 203 how The word ‘ebullioscopy’
a hotter temperature promotes faster cooking.) The salt causes an comes from the Latin
elevation of boiling point, which is another colligative property. We (e)bulirre, meaning
call the determination of such an elevation ebullioscopy. ‘bubbles’ or ‘bubbly’.
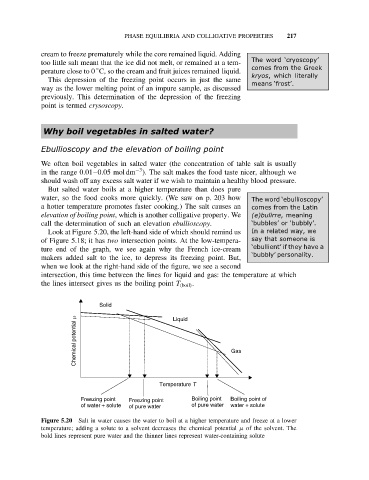
Look at Figure 5.20, the left-hand side of which should remind us In a related way, we
of Figure 5.18; it has two intersection points. At the low-tempera- say that someone is
ture end of the graph, we see again why the French ice-cream ‘ebullient’ if they have a
‘bubbly’ personality.
makers added salt to the ice, to depress its freezing point. But,
when we look at the right-hand side of the figure, we see a second
intersection, this time between the lines for liquid and gas: the temperature at which
the lines intersect gives us the boiling point T (boil) .
Solid Liquid
Chemical potential m Gas
Temperature T
Freezing point Freezing point Boiling point Boiling point of
of water + solute of pure water of pure water water + solute
Figure 5.20 Salt in water causes the water to boil at a higher temperature and freeze at a lower
temperature; adding a solute to a solvent decreases the chemical potential µ of the solvent. The
bold lines represent pure water and the thinner lines represent water-containing solute