Page 279 - Physical chemistry understanding our chemical world
P. 279
246 ACIDS AND BASES
concentration of the solvated protons in vinegar lies in the range
Care:the ‘H’inpH 10 −4 –10 −5 mol dm .
−3
derives from the sym- Between these two acids, there is up to a million-fold differ-
bol for hydrogen, and ence in the number of solvated protons per litre. We cannot cope
is always given a big with the unwieldy magnitude of this difference and tend to talk
letter. The ‘p’ is a math-
ematical operator, and instead in terms of the logarithm of the concentration. To this end,
is always small. we introduce a new concept: the pH. This is defined mathemati-
cally as ‘minus the logarithm (to the base ten) of the hydrogen ion
concentration’:
−3
An acid’s pH is defined pH =− log [H /mol dm ] (6.20)
+
10
as minus the loga-
rithm (to the base ten) The concentrations of bench acids in an undergraduate labora-
of the hydrogen ion tory are generally less than 1 mol dm , so by corollary the minus
−3
concentration.
sign to Equation (6.20) suggests we generally work with positive
values of pH. Only if the solution has a concentration greater than
The ‘p’ in Equation 1 mol dm −3 will the pH be negative. Contrary to popular belief,
(6.20) is the mathe- a negative pH is not impossible. (Try inserting a concentration of
matical operator 2.0 mol dm −3 into Equation (6.20) and see what happens!)
+
‘− log ’ of something. Notice how we generally infer the solvated proton,H 3 O , each
10
pH means we have time we write a concentration as [H ], which helps explain why
+
applied the operator ‘p’ the concept of pH is rarely useful when considering acids dis-
to [H ]. The p is short solved in non-aqueous solvents. When comparing the battery acid
+
for potenz,German for
with the bench acid, we say that the battery acid has a lower pH
power.
than does the bench acid, because the number of solvated protons
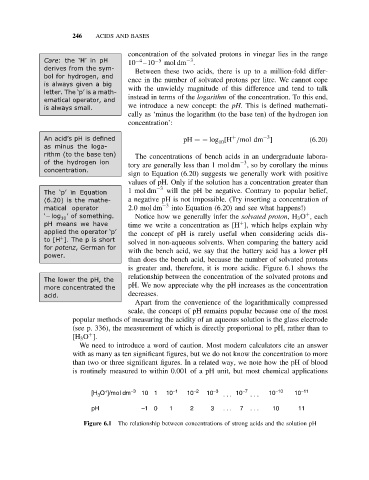
is greater and, therefore, it is more acidic. Figure 6.1 shows the
relationship between the concentration of the solvated protons and
Thelower thepH, the
more concentrated the pH. We now appreciate why the pH increases as the concentration
acid. decreases.
Apart from the convenience of the logarithmically compressed
scale, the concept of pH remains popular because one of the most
popular methods of measuring the acidity of an aqueous solution is the glass electrode
(see p. 336), the measurement of which is directly proportional to pH, rather than to
+
[H 3 O ].
We need to introduce a word of caution. Most modern calculators cite an answer
with as many as ten significant figures, but we do not know the concentration to more
than two or three significant figures. In a related way, we note how the pH of blood
is routinely measured to within 0.001 of a pH unit, but most chemical applications
+ −3 −1 −2 −3 −7 −10 −11
[H O ]/mol dm 10 1 10 10 10 . . . 10 . . . 10 10
3
pH −1 0 1 2 3 . . . 7 . . . 10 11
Figure 6.1 The relationship between concentrations of strong acids and the solution pH