Page 82 - Physical chemistry understanding our chemical world
P. 82
PHYSICAL AND MOLECULAR INTERACTIONS 49
Why is petrol a liquid at room temperature but butane
is a gas?
The magnitude of London dispersion forces
The major component of the petrol that fuels a car is octane, present as a mixture of
various isomers. It is liquid at room temperature because its boiling point temperature
◦
◦
T (boil) is about 125.7 C. The methane gas powering an oven has a T (boil) =−162.5 C
◦
and the butane propellant in a can of air freshener has T (boil) =−0.5 C. Octadecane
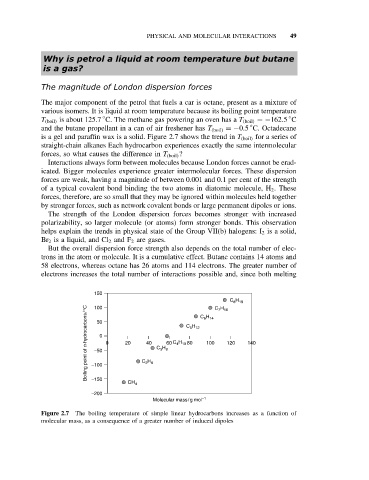
is a gel and paraffin wax is a solid. Figure 2.7 shows the trend in T (boil) for a series of
straight-chain alkanes Each hydrocarbon experiences exactly the same intermolecular
forces, so what causes the difference in T (boil) ?
Interactions always form between molecules because London forces cannot be erad-
icated. Bigger molecules experience greater intermolecular forces. These dispersion
forces are weak, having a magnitude of between 0.001 and 0.1 per cent of the strength
of a typical covalent bond binding the two atoms in diatomic molecule, H 2 . These
forces, therefore, are so small that they may be ignored within molecules held together
by stronger forces, such as network covalent bonds or large permanent dipoles or ions.
The strength of the London dispersion forces becomes stronger with increased
polarizability, so larger molecule (or atoms) form stronger bonds. This observation
helps explain the trends in physical state of the Group VII(b) halogens: I 2 is a solid,
Br 2 is a liquid, and Cl 2 and F 2 are gases.
But the overall dispersion force strength also depends on the total number of elec-
trons in the atom or molecule. It is a cumulative effect. Butane contains 14 atoms and
58 electrons, whereas octane has 26 atoms and 114 electrons. The greater number of
electrons increases the total number of interactions possible and, since both melting
150
C 8 H 18
100
Boiling point of n-hydrocarbons/°C −50 0 0 20 C 2 H 6 C 3 H 8 60 C 4 H 10 C 5 H 12 100 120 140
C 7 H 16
C 6 H 14
50
80
40
−100
−150
CH 4
−200
Molecular mass/g mol −1
Figure 2.7 The boiling temperature of simple linear hydrocarbons increases as a function of
molecular mass, as a consequence of a greater number of induced dipoles