Page 81 - Physical chemistry understanding our chemical world
P. 81
48 INTRODUCING INTERACTIONS AND BONDS
− + − +
d d d d
Electrons Atomic
nucleus
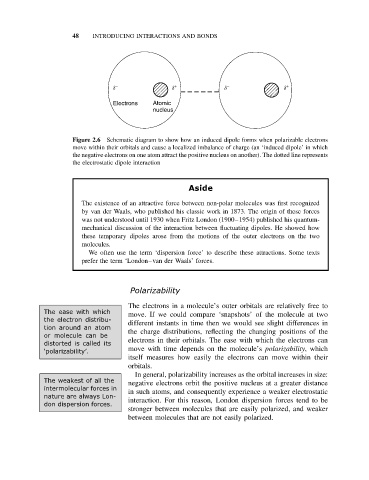
Figure 2.6 Schematic diagram to show how an induced dipole forms when polarizable electrons
move within their orbitals and cause a localized imbalance of charge (an ‘induced dipole’ in which
the negative electrons on one atom attract the positive nucleus on another). The dotted line represents
the electrostatic dipole interaction
Aside
The existence of an attractive force between non-polar molecules was first recognized
by van der Waals, who published his classic work in 1873. The origin of these forces
was not understood until 1930 when Fritz London (1900–1954) published his quantum-
mechanical discussion of the interaction between fluctuating dipoles. He showed how
these temporary dipoles arose from the motions of the outer electrons on the two
molecules.
We often use the term ‘dispersion force’ to describe these attractions. Some texts
prefer the term ‘London–van der Waals’ forces.
Polarizability
The electrons in a molecule’s outer orbitals are relatively free to
The ease with which move. If we could compare ‘snapshots’ of the molecule at two
the electron distribu- different instants in time then we would see slight differences in
tion around an atom
the charge distributions, reflecting the changing positions of the
or molecule can be
distorted is called its electrons in their orbitals. The ease with which the electrons can
move with time depends on the molecule’s polarizability, which
‘polarizability’.
itself measures how easily the electrons can move within their
orbitals.
In general, polarizability increases as the orbital increases in size:
The weakest of all the
negative electrons orbit the positive nucleus at a greater distance
intermolecular forces in in such atoms, and consequently experience a weaker electrostatic
nature are always Lon-
don dispersion forces. interaction. For this reason, London dispersion forces tend to be
stronger between molecules that are easily polarized, and weaker
between molecules that are not easily polarized.