Page 263 - Physical Chemistry
P. 263
lev38627_ch08.qxd 3/14/08 12:54 PM Page 244
CHAPTER
8 Real Gases
CHAPTER OUTLINE
8.1 Compression Factors 8.1 COMPRESSION FACTORS
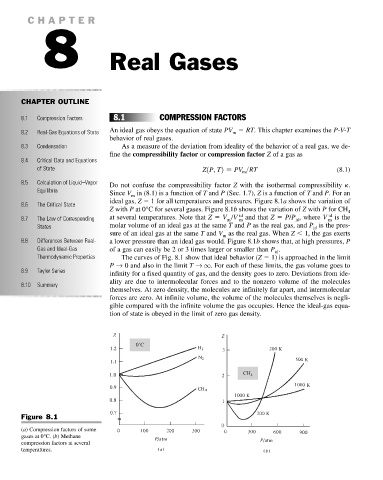
8.2 Real-Gas Equations of State An ideal gas obeys the equation of state PV RT. This chapter examines the P-V-T
m
behavior of real gases.
8.3 Condensation As a measure of the deviation from ideality of the behavior of a real gas, we de-
fine the compressibility factor or compression factor Z of a gas as
8.4 Critical Data and Equations
of State Z1P, T2 PV >RT (8.1)
m
8.5 Calculation of Liquid–Vapor Do not confuse the compressibility factor Z with the isothermal compressibility k.
Equilibria Since V in (8.1) is a function of T and P (Sec. 1.7), Z is a function of T and P. For an
m
ideal gas, Z 1 for all temperatures and pressures. Figure 8.1a shows the variation of
8.6 The Critical State
Z with P at 0°C for several gases. Figure 8.1b shows the variation of Z with P for CH 4
8.7 The Law of Corresponding at several temperatures. Note that Z V /V id and that Z P/P , where V id is the
m
id
m
m
States molar volume of an ideal gas at the same T and P as the real gas, and P is the pres-
id
sure of an ideal gas at the same T and V as the real gas. When Z 1, the gas exerts
m
8.8 Differences Between Real- a lower pressure than an ideal gas would. Figure 8.1b shows that, at high pressures, P
Gas and Ideal-Gas of a gas can easily be 2 or 3 times larger or smaller than P .
id
Thermodynamic Properties The curves of Fig. 8.1 show that ideal behavior (Z 1) is approached in the limit
P → 0 and also in the limit T → q. For each of these limits, the gas volume goes to
8.9 Taylor Series infinity for a fixed quantity of gas, and the density goes to zero. Deviations from ide-
ality are due to intermolecular forces and to the nonzero volume of the molecules
8.10 Summary
themselves. At zero density, the molecules are infinitely far apart, and intermolecular
forces are zero. At infinite volume, the volume of the molecules themselves is negli-
gible compared with the infinite volume the gas occupies. Hence the ideal-gas equa-
tion of state is obeyed in the limit of zero gas density.
0˚C
CH 4
Figure 8.1
(a) Compression factors of some
gases at 0°C. (b) Methane
compression factors at several
temperatures.