Page 268 - Physical Chemistry
P. 268
lev38627_ch08.qxd 3/14/08 12:54 PM Page 249
249
TABLE 8.1 Section 8.4
Critical Data and Equations of State
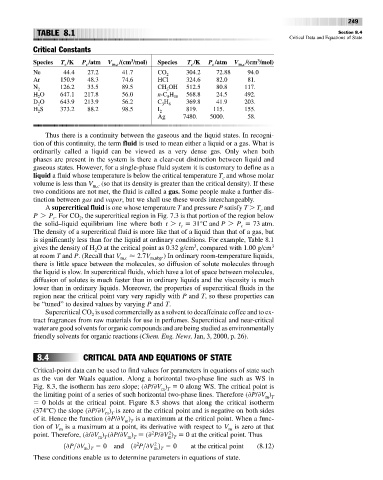
Critical Constants
3
3
Species T /K P /atm V m,c /(cm /mol) Species T /K P /atm V m,c /(cm /mol)
c
c
c
c
Ne 44.4 27.2 41.7 CO 2 304.2 72.88 94.0
Ar 150.9 48.3 74.6 HCl 324.6 82.0 81.
N 2 126.2 33.5 89.5 CH OH 512.5 80.8 117.
3
H O 647.1 217.8 56.0 n-C H 18 568.8 24.5 492.
2
8
D O 643.9 213.9 56.2 C H 8 369.8 41.9 203.
2
3
H S 373.2 88.2 98.5 I 2 819. 115. 155.
2
Ag 7480. 5000. 58.
Thus there is a continuity between the gaseous and the liquid states. In recogni-
tion of this continuity, the term fluid is used to mean either a liquid or a gas. What is
ordinarily called a liquid can be viewed as a very dense gas. Only when both
phases are present in the system is there a clear-cut distinction between liquid and
gaseous states. However, for a single-phase fluid system it is customary to define as a
liquid a fluid whose temperature is below the critical temperature T and whose molar
c
volume is less than V (so that its density is greater than the critical density). If these
m,c
two conditions are not met, the fluid is called a gas. Some people make a further dis-
tinction between gas and vapor, but we shall use these words interchangeably.
A supercritical fluid is one whose temperature T and pressure P satisfy T T and
c
P 7 P . For CO , the supercritical region in Fig. 7.3 is that portion of the region below
2
c
the solid–liquid equilibrium line where both t 7 t 31°C and P 7 P 73 atm.
c
c
The density of a supercritical fluid is more like that of a liquid than that of a gas, but
is significantly less than for the liquid at ordinary conditions. For example, Table 8.1
3
gives the density of H O at the critical point as 0.32 g/cm , compared with 1.00 g/cm 3
2
at room T and P. (Recall that V m,c 2.7V m,nbp . ) In ordinary room-temperature liquids,
there is little space between the molecules, so diffusion of solute molecules through
the liquid is slow. In supercritical fluids, which have a lot of space between molecules,
diffusion of solutes is much faster than in ordinary liquids and the viscosity is much
lower than in ordinary liquids. Moreover, the properties of supercritical fluids in the
region near the critical point vary very rapidly with P and T, so these properties can
be “tuned” to desired values by varying P and T.
Supercritical CO is used commercially as a solvent to decaffeinate coffee and to ex-
2
tract fragrances from raw materials for use in perfumes. Supercritical and near-critical
water are good solvents for organic compounds and are being studied as environmentally
friendly solvents for organic reactions (Chem. Eng. News, Jan, 3, 2000, p. 26).
8.4 CRITICAL DATA AND EQUATIONS OF STATE
Critical-point data can be used to find values for parameters in equations of state such
as the van der Waals equation. Along a horizontal two-phase line such as WS in
Fig. 8.3, the isotherm has zero slope; ( P/ V ) 0 along WS. The critical point is
m T
the limiting point of a series of such horizontal two-phase lines. Therefore ( P/ V )
m T
0 holds at the critical point. Figure 8.3 shows that along the critical isotherm
(374°C) the slope ( P/ V ) is zero at the critical point and is negative on both sides
m T
of it. Hence the function ( P/ V ) is a maximum at the critical point. When a func-
m T
tion of V is a maximum at a point, its derivative with respect to V is zero at that
m m
2
2
point. Therefore, ( / V ) ( P/ V ) ( P/ V ) 0 at the critical point. Thus
m T m T m T
2
2
10P>0V 2 0 and 10 P>0V 2 0 at the critical point (8.12)
m T
m T
These conditions enable us to determine parameters in equations of state.