Page 272 - Physical Chemistry
P. 272
lev38627_ch08.qxd 3/14/08 12:54 PM Page 253
253
We have to solve the three simultaneous equations (8.25) and (8.26) for the three un- Section 8.5
v
l
knowns: the vapor pressure P and the liquid and vapor molar volumes V and V . Calculation of Liquid–Vapor
m
m
Equilibria
Example 8.1 shows how this is done using the Excel spreadsheet.
EXAMPLE 8.1 Prediction of vapor pressure from an equation of state
Use the Redlich–Kwong equation to estimate the vapor pressure and the satu-
rated liquid and vapor molar volumes of C H at 25°C.
8
3
Equations (8.20) and (8.21) and the critical constants in Table 8.1 give the
8
6
propane Redlich–Kwong constants as a 1.80 10 cm atm K 1/2 mol 2 and
7
3
b 62.7 cm /mol. To get initial estimates of the unknowns (which are needed to
use the Solver), we graph the propane 25°C Redlich–Kwong isotherm. The val-
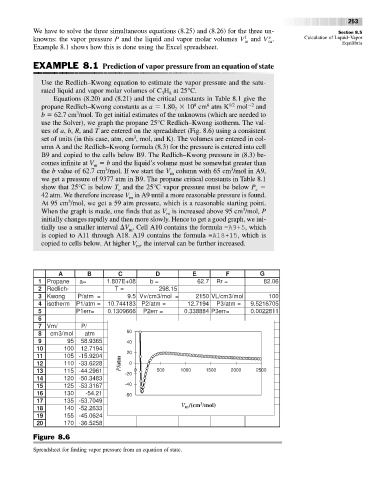
ues of a, b, R, and T are entered on the spreadsheet (Fig. 8.6) using a consistent
3
set of units (in this case, atm, cm , mol, and K). The volumes are entered in col-
umn A and the Redlich–Kwong formula (8.3) for the pressure is entered into cell
B9 and copied to the cells below B9. The Redlich–Kwong pressure in (8.3) be-
comes infinite at V b and the liquid’s volume must be somewhat greater than
m
3
3
the b value of 62.7 cm /mol. If we start the V column with 65 cm /mol in A9,
m
we get a pressure of 9377 atm in B9. The propane critical constants in Table 8.1
show that 25°C is below T and the 25°C vapor pressure must be below P
c
c
42 atm. We therefore increase V in A9 until a more reasonable pressure is found.
m
3
At 95 cm /mol, we get a 59 atm pressure, which is a reasonable starting point.
3
When the graph is made, one finds that as V is increased above 95 cm /mol, P
m
initially changes rapidly and then more slowly. Hence to get a good graph, we ini-
tially use a smaller interval V . Cell A10 contains the formula =A9+5, which
m
is copied to A11 through A18. A19 contains the formula =A18+15, which is
copied to cells below. At higher V , the interval can be further increased.
m
A B C D E F G
1 Propane a= 1.807E+08 b = 62.7 Rr = 82.06
2 Redlich- T = 298.15
3 Kwong P/atm = 9.5 Vv/cm3/mol = 2150 VL/cm3/mol 100
4 isotherm P1/atm = 10.744183 P2/atm = 12.7194 P3/atm = 9.5216705
5 P1err= 0.1309666 P2err = 0.338884 P3err= 0.0022811
6
7 Vm/ P/
60
8 cm3/mol atm
9 95 58.9365 40
10 100 12.7194
20
11 105 -15.9204
12 110 -33.6228 P/atm 0
13 115 -44.2961 0 500 1000 1500 2000 2500
-20
14 120 -50.3483
15 125 -53.3167 -40
16 130 -54.21 -60
17 135 -53.7049
3
V m /(cm /mol)
18 140 -52.2633
19 155 -45.0624
20 170 -36.5258
Figure 8.6
Spreadsheet for finding vapor pressure from an equation of state.