Page 275 - Physical Chemistry
P. 275
lev38627_ch08.qxd 3/14/08 12:54 PM Page 256
256
Chapter 8
Real Gases
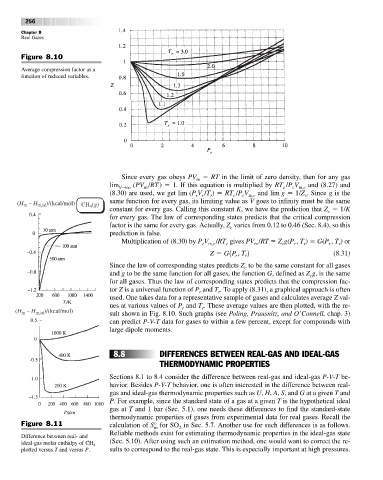
Figure 8.10
Average compression factor as a
function of reduced variables.
Since every gas obeys PV RT in the limit of zero density, then for any gas
m
lim (PV /RT ) 1. If this equation is multiplied by RT /P V and (8.27) and
V→q m c c m,c
(8.30) are used, we get lim (P V /T ) RT /P V and lim g 1/Z . Since g is the
c m,c
r r
c
c
r
(H – H m,id )/(kcal/mol) CH (g) same function for every gas, its limiting value as V goes to infinity must be the same
CH (g)
m
4 4
constant for every gas. Calling this constant K, we have the prediction that Z 1/K
c
for every gas. The law of corresponding states predicts that the critical compression
factor is the same for every gas. Actually, Z varies from 0.12 to 0.46 (Sec. 8.4), so this
c
prediction is false.
Multiplication of (8.30) by P V /RT gives PV /RT Z g(P , T ) G(P , T ) or
r
m
r
r
c
r
c
c m,c
Z G1P , T 2 (8.31)
r
r
Since the law of corresponding states predicts Z to be the same constant for all gases
c
and g to be the same function for all gases, the function G, defined as Z g, is the same
c
for all gases. Thus the law of corresponding states predicts that the compression fac-
tor Z is a universal function of P and T . To apply (8.31), a graphical approach is often
r
r
used. One takes data for a representative sample of gases and calculates average Z val-
ues at various values of P and T . These average values are then plotted, with the re-
r
r
(H – H m,id )/(kcal/mol) sult shown in Fig. 8.10. Such graphs (see Poling, Prausnitz, and O’Connell, chap. 3)
m
can predict P-V-T data for gases to within a few percent, except for compounds with
large dipole moments.
8.8 DIFFERENCES BETWEEN REAL-GAS AND IDEAL-GAS
THERMODYNAMIC PROPERTIES
Sections 8.1 to 8.4 consider the difference between real-gas and ideal-gas P-V-T be-
havior. Besides P-V-T behavior, one is often interested in the difference between real-
gas and ideal-gas thermodynamic properties such as U, H, A, S, and G at a given T and
P. For example, since the standard state of a gas at a given T is the hypothetical ideal
gas at T and 1 bar (Sec. 5.1), one needs these differences to find the standard-state
thermodynamic properties of gases from experimental data for real gases. Recall the
Figure 8.11 calculation of S° for SO in Sec. 5.7. Another use for such differences is as follows.
m 2
Reliable methods exist for estimating thermodynamic properties in the ideal-gas state
Difference between real- and
ideal-gas molar enthalpy of CH 4 (Sec. 5.10). After using such an estimation method, one would want to correct the re-
plotted versus T and versus P. sults to correspond to the real-gas state. This is especially important at high pressures.