Page 291 - Physical Chemistry
P. 291
lev38627_ch09.qxd 3/14/08 1:31 PM Page 272
272
Chapter 9 Mixing quantities such as mix V, mix H, and mix S tell us about intermolecular in-
Solutions teractions in the solution as compared with those in the pure components.
Unfortunately, interpretation of mixing quantities of liquids in terms of molecular
interactions is difficult; see Rowlinson and Swinton, chap. 5, for examples.
A(l, P) B(l, P)
Experimental Determination of Mixing Quantities
1
V is easily found from density measurements on the solution and the pure com-
mix
ponents or from direct measurement of the volume change on isothermal mixing of
A(l, P*) B(l, P*)
A
B
the components. H at constant T and P is easily measured in a constant-pressure
mix
calorimeter.
2
How do we get G? G is calculated from vapor-pressure measurements.
mix mix
A(g, P*) B(g, P*) One measures the partial pressures P and P of A and B in the vapor in equilibrium
B
A
A
B
with the solution and measures the vapor pressures P* and P* of pure A and pure B
A B
3 at the temperature of the solution. The hypothetical isothermal path of Fig. 9.6 starts
with the pure liquids A and B at T and P and ends with the liquid solution at T and P.
A(g, P ) B(g, P ) Therefore G for this six-step process equals G. One uses thermodynamic rela-
A
B
mix
tions to express G of each step in terms of P , P , P*, and P*, thereby obtaining
A
B
A
B
4 G in terms of these vapor pressures. If the gases A and B are assumed ideal and
mix
the slight changes in G in steps 1 and 6 are neglected, the result is (Prob. 9.64)
(A B)(g, P P )
A
B
¢ G n RT ln1P >P*2 n RT ln1P >P *2
mix A A A B B B
5
S is found from G and H using G H T S.
mix mix mix mix mix mix
(A B)(l, P P )
B
A
6
9.4 DETERMINATION OF PARTIAL MOLAR QUANTITIES
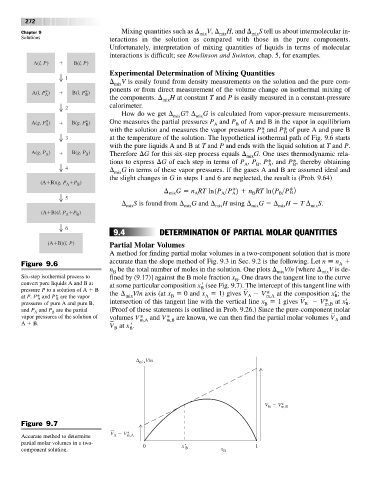
(A B)(l, P) Partial Molar Volumes
A method for finding partial molar volumes in a two-component solution that is more
accurate than the slope method of Fig. 9.3 in Sec. 9.2 is the following. Let n n
Figure 9.6 A
n be the total number of moles in the solution. One plots V/n [where V is de-
B mix mix
Six-step isothermal process to fined by (9.17)] against the B mole fraction x . One draws the tangent line to the curve
B
convert pure liquids A and B at at some particular composition x (see Fig. 9.7). The intercept of this tangent line with
pressure P to a solution of A B B
x¿
the ¢ V/n axis (at x 0 and x 1) gives V V* at the composition ; the
at P. P* and P* are the vapor mix B A A m,A B
A
B
x
¿
pressures of pure A and pure B, intersection of this tangent line with the vertical line x 1 gives V B V* at .
B
B
m,B
and P and P are the partial (Proof of these statements is outlined in Prob. 9.26.) Since the pure-component molar
B
A
vapor pressures of the solution of volumes V* and V* are known, we can then find the partial molar volumes V A and
m,B
m,A
A B. at x .
V
B B
∆ mix V/n
V V* m,B
B
Figure 9.7
V V*
Accurate method to determine A m,A
partial molar volumes in a two- 0 x′ 1
component solution. B x B