Page 319 - Physical Chemistry
P. 319
lev38627_ch10.qxd 3/14/08 1:07 PM Page 300
300
Chapter 10 TABLE 10.1
Nonideal Solutions
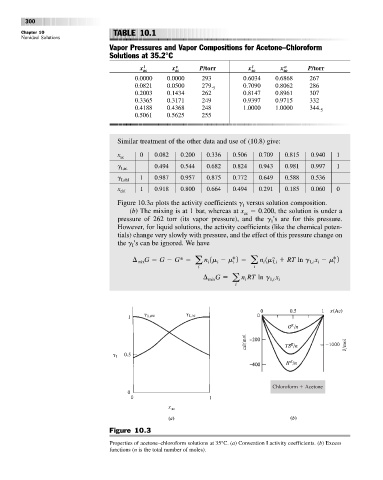
Vapor Pressures and Vapor Compositions for Acetone–Chloroform
Solutions at 35.2°C
v
l
v
l
x ac x ac P/torr x ac x ac P/torr
0.0000 0.0000 293 0.6034 0.6868 267
0.0821 0.0500 279. 5 0.7090 0.8062 286
0.2003 0.1434 262 0.8147 0.8961 307
0.3365 0.3171 249 0.9397 0.9715 332
0.4188 0.4368 248 1.0000 1.0000 344. 5
0.5061 0.5625 255
Similar treatment of the other data and use of (10.8) give:
x 0 0.082 0.200 0.336 0.506 0.709 0.815 0.940 1
ac
g 0.494 0.544 0.682 0.824 0.943 0.981 0.997 1
I,ac
g 1 0.987 0.957 0.875 0.772 0.649 0.588 0.536
I,chl
x 1 0.918 0.800 0.664 0.494 0.291 0.185 0.060 0
chl
Figure 10.3a plots the activity coefficients g versus solution composition.
I
(b) The mixing is at 1 bar, whereas at x 0.200, the solution is under a
ac
pressure of 262 torr (its vapor pressure), and the g ’s are for this pressure.
I
However, for liquid solutions, the activity coefficients (like the chemical poten-
tials) change very slowly with pressure, and the effect of this pressure change on
the g ’s can be ignored. We have
I
¢ mix G G G* a n 1m m*2 a n 1m° RT ln g x m*2
i
i
I,i i
i
I,i
i
i
i i
¢ mix G a n RT ln g x
i
I,i i
i
Chloroform Acetone
Figure 10.3
Properties of acetone–chloroform solutions at 35°C. (a) Convention I activity coefficients. (b) Excess
functions (n is the total number of moles).