Page 265 - Pipeline Rules of Thumb Handbook
P. 265
252 Pipeline Rules of Thumb Handbook
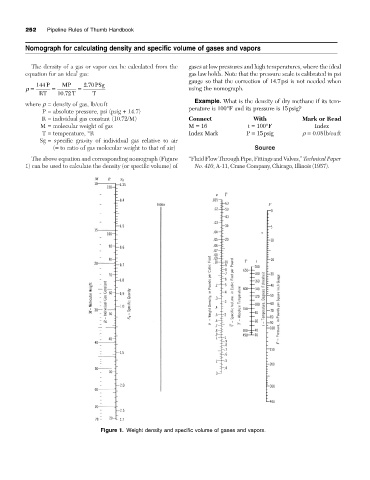
Nomograph for calculating density and specific volume of gases and vapors
The density of a gas or vapor can be calculated from the gases at low pressures and high temperatures, where the ideal
equation for an ideal gas: gas law holds. Note that the pressure scale is calibrated in psi
gauge so that the correction of 14.7psi is not needed when
144 P MP 270PSg
.
r = = = using the nomograph.
RT 10 72 T T
.
Example. What is the density of dry methane if its tem-
where r = density of gas, lb/cuft
perature is 100°F and its pressure is 15psig?
P = absolute pressure, psi (psig + 14.7)
R = individual gas constant (10.72/M) Connect With Mark or Read
M = molecular weight of gas M = 16 t = 100°F Index
T = temperature, °R Index Mark P = 15psig r = 0.08lb/cuft
Sg = specific gravity of individual gas relative to air
(= to ratio of gas molecular weight to that of air) Source
The above equation and corresponding nomograph (Figure “Fluid Flow Through Pipe, Fittings and Valves,” Technical Paper
1) can be used to calculate the density (or specific volume) of No. 410, A-11, Crane Company, Chicago, Illinois (1957).
Figure 1. Weight density and specific volume of gases and vapors.