Page 145 - Plant design and economics for chemical engineers
P. 145
120 PLANT DESIGN AND ECONOMICS FOR CHEMICAL ENGINEERS
n
By-products
Condensed
steam
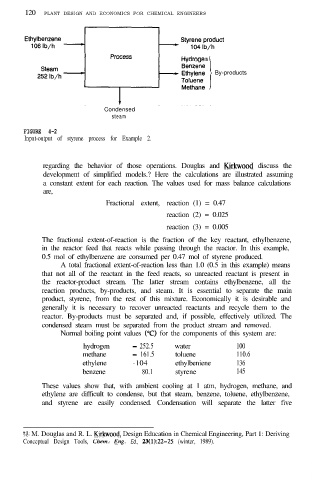
FIGURE 4-2
Input-output of styrene process for Example 2.
regarding the behavior of those operations. Douglas and Kirkwood discuss the
development of simplified models.? Here the calculations are illustrated assuming
a constant extent for each reaction. The values used for mass balance calculations
are,
Fractional extent, reaction (1) = 0.47
reaction (2) = 0.025
reaction (3) = 0.005
The fractional extent-of-reaction is the fraction of the key reactant, ethylbenzene,
in the reactor feed that reacts while passing through the reactor. In this example,
0.5 mol of ethylbenzene are consumed per 0.47 mol of styrene produced.
A total fractional extent-of-reaction less than 1.0 (0.5 in this example) means
that not all of the reactant in the feed reacts, so unreacted reactant is present in
the reactor-product stream. The latter stream contains ethylbenzene, all the
reaction products, by-products, and steam. It is essential to separate the main
product, styrene, from the rest of this mixture. Economically it is desirable and
generally it is necessary to recover unreacted reactants and recycle them to the
reactor. By-products must be separated and, if possible, effectively utilized. The
condensed steam must be separated from the product stream and removed.
Normal boiling point values (OC) for the components of this system are:
hydrogen - 252.5 water 100
methane - 161.5 toluene 110.6
ethylene -104 ethylbeniene 136
benzene 80.1 styrene 145
These values show that, with ambient cooling at 1 atm, hydrogen, methane, and
ethylene are difficult to condense, but that steam, benzene, toluene, ethylbenzene,
and styrene are easily condensed. Condensation will separate the latter five
tJ. M. Douglas and R. L. Kirkwood, Design Education in Chemical Engineering, Part 1: Deriving
Conceptual Design Tools, Gem. Eng. Ed., 23(1):22-25 (winter, 1989).