Page 41 - Plant design and economics for chemical engineers
P. 41
24 PLANT DESIGN AND ECONOMICS FOR CHEM!CAL ENGINEERS
Dodecene
R e a c t 0r
(olkylot or)
S etttler .L L- L-
Aicl, sludge
- -NaOH Spray
Yiactor I \ I
Y
Detergent
product
“Builders”
H e a v y Spent
alkyloted acid
h y d r o c a r b o n s
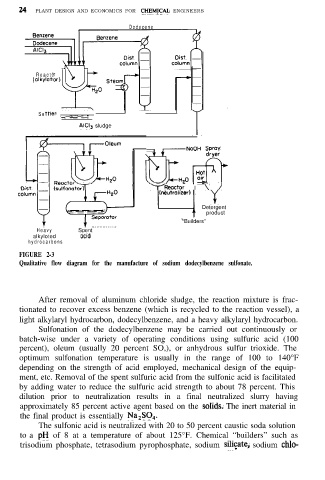
FIGURE 2-3
Qualitative flow diagram for the manufacture of sodium dodecylbenzene sulfonate.
After removal of aluminum chloride sludge, the reaction mixture is frac-
tionated to recover excess benzene (which is recycled to the reaction vessel), a
light alkylaryl hydrocarbon, dodecylbenzene, and a heavy alkylaryl hydrocarbon.
Sulfonation of the dodecylbenzene may be carried out continuously or
batch-wise under a variety of operating conditions using sulfuric acid (100
percent), oleum (usually 20 percent SO,), or anhydrous sulfur trioxide. The
optimum sulfonation temperature is usually in the range of 100 to 140°F
depending on the strength of acid employed, mechanical design of the equip-
ment, etc. Removal of the spent sulfuric acid from the sulfonic acid is facilitated
by adding water to reduce the sulfuric acid strength to about 78 percent. This
dilution prior to neutralization results in a final neutralized slurry having
approximately 85 percent active agent based on the sohds. The inert material in
the final product is essentially Na,SO,.
The sulfonic acid is neutralized with 20 to 50 percent caustic soda solution
to a pH of 8 at a temperature of about 125°F. Chemical “builders” such as
trisodium phosphate, tetrasodium pyrophosphate, sodium silitate, sodium chlo-