Page 295 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 295
264 Polymer-based Nanocomposites for Energy and Environmental Applications
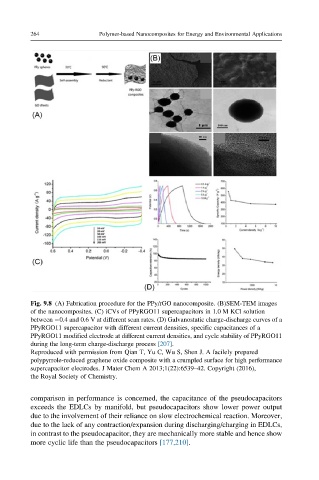
Fig. 9.8 (A) Fabrication procedure for the PPy/rGO nanocomposite. (B)SEM-TEM images
of the nanocomposites. (C) iCVs of PPyRGO11 supercapacitors in 1.0 M KCl solution
between 0.4 and 0.6 V at different scan rates. (D) Galvanostatic charge-discharge curves of a
PPyRGO11 supercapacitor with different current densities, specific capacitances of a
PPyRGO11 modified electrode at different current densities, and cycle stability of PPyRGO11
during the long-term charge-discharge process [207].
Reproduced with permission from Qian T, Yu C, Wu S, Shen J. A facilely prepared
polypyrrole-reduced graphene oxide composite with a crumpled surface for high performance
supercapacitor electrodes. J Mater Chem A 2013;1(22):6539–42. Copyright (2016),
the Royal Society of Chemistry.
comparison in performance is concerned, the capacitance of the pseudocapacitors
exceeds the EDLCs by manifold, but pseudocapacitors show lower power output
due to the involvement of their reliance on slow electrochemical reaction. Moreover,
due to the lack of any contraction/expansion during discharging/charging in EDLCs,
in contrast to the pseudocapacitor, they are mechanically more stable and hence show
more cyclic life than the pseudocapacitors [177,210].