Page 297 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 297
266 Polymer-based Nanocomposites for Energy and Environmental Applications
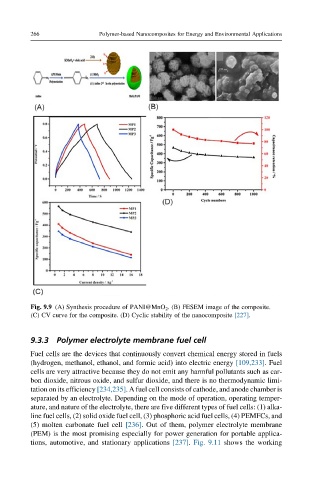
Fig. 9.9 (A) Synthesis procedure of PANI@MnO 2 . (B) FESEM image of the composite.
(C) CV curve for the composite. (D) Cyclic stability of the nanocomposite [227].
9.3.3 Polymer electrolyte membrane fuel cell
Fuel cells are the devices that continuously convert chemical energy stored in fuels
(hydrogen, methanol, ethanol, and formic acid) into electric energy [109,233]. Fuel
cells are very attractive because they do not emit any harmful pollutants such as car-
bon dioxide, nitrous oxide, and sulfur dioxide, and there is no thermodynamic limi-
tation on its efficiency [234,235]. A fuel cell consists of cathode, and anode chamber is
separated by an electrolyte. Depending on the mode of operation, operating temper-
ature, and nature of the electrolyte, there are five different types of fuel cells: (1) alka-
line fuel cells, (2) solid oxide fuel cell, (3) phosphoric acid fuel cells, (4) PEMFCs, and
(5) molten carbonate fuel cell [236]. Out of them, polymer electrolyte membrane
(PEM) is the most promising especially for power generation for portable applica-
tions, automotive, and stationary applications [237]. Fig. 9.11 shows the working