Page 299 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 299
268 Polymer-based Nanocomposites for Energy and Environmental Applications
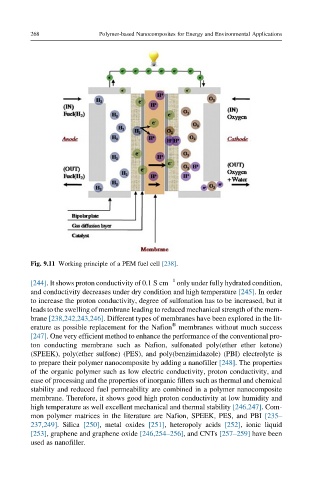
Fig. 9.11 Working principle of a PEM fuel cell [238].
[244]. It shows proton conductivity of 0.1 S cm 1 only under fully hydrated condition,
and conductivity decreases under dry condition and high temperature [245]. In order
to increase the proton conductivity, degree of sulfonation has to be increased, but it
leads to the swelling of membrane leading to reduced mechanical strength of the mem-
brane [238,242,243,246]. Different types of membranes have been explored in the lit-
®
erature as possible replacement for the Nafion membranes without much success
[247]. One very efficient method to enhance the performance of the conventional pro-
ton conducting membrane such as Nafion, sulfonated poly(ether ether ketone)
(SPEEK), poly(ether sulfone) (PES), and poly(benzimidazole) (PBI) electrolyte is
to prepare their polymer nanocomposite by adding a nanofiller [248]. The properties
of the organic polymer such as low electric conductivity, proton conductivity, and
ease of processing and the properties of inorganic fillers such as thermal and chemical
stability and reduced fuel permeability are combined in a polymer nanocomposite
membrane. Therefore, it shows good high proton conductivity at low humidity and
high temperature as well excellent mechanical and thermal stability [246,247]. Com-
mon polymer matrices in the literature are Nafion, SPEEK, PES, and PBI [235–
237,249]. Silica [250], metal oxides [251], heteropoly acids [252], ionic liquid
[253], graphene and graphene oxide [246,254–256], and CNTs [257–259] have been
used as nanofiller.