Page 298 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 298
Polymer nanocomposite materials in energy storage: Properties and applications 267
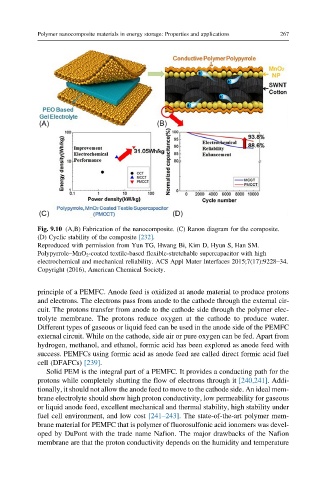
Fig. 9.10 (A,B) Fabrication of the nanocomposite. (C) Ranon diagram for the composite.
(D) Cyclic stability of the composite [232].
Reproduced with permission from Yun TG, Hwang Bi, Kim D, Hyun S, Han SM.
Polypyrrole–MnO 2 -coated textile-based flexible-stretchable supercapacitor with high
electrochemical and mechanical reliability. ACS Appl Mater Interfaces 2015;7(17):9228–34.
Copyright (2016), American Chemical Society.
principle of a PEMFC. Anode feed is oxidized at anode material to produce protons
and electrons. The electrons pass from anode to the cathode through the external cir-
cuit. The protons transfer from anode to the cathode side through the polymer elec-
trolyte membrane. The protons reduce oxygen at the cathode to produce water.
Different types of gaseous or liquid feed can be used in the anode side of the PEMFC
external circuit. While on the cathode, side air or pure oxygen can be fed. Apart from
hydrogen, methanol, and ethanol, formic acid has been explored as anode feed with
success. PEMFCs using formic acid as anode feed are called direct formic acid fuel
cell (DFAFCs) [239].
Solid PEM is the integral part of a PEMFC. It provides a conducting path for the
protons while completely shutting the flow of electrons through it [240,241]. Addi-
tionally, it should not allow the anode feed to move to the cathode side. An ideal mem-
brane electrolyte should show high proton conductivity, low permeability for gaseous
or liquid anode feed, excellent mechanical and thermal stability, high stability under
fuel cell environment, and low cost [241–243]. The state-of-the-art polymer mem-
brane material for PEMFC that is polymer of fluorosulfonic acid ionomers was devel-
oped by DuPont with the trade name Nafion. The major drawbacks of the Nafion
membrane are that the proton conductivity depends on the humidity and temperature