Page 319 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 319
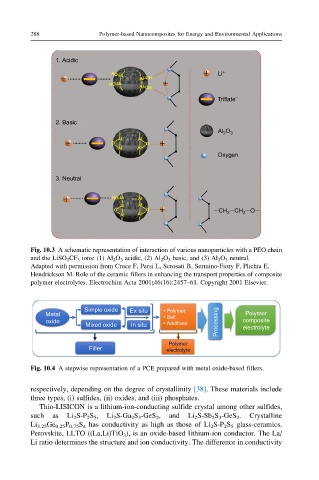
288 Polymer-based Nanocomposites for Energy and Environmental Applications
1. Acidic
Li +
−
Triflate
2. Basic
Al O 3
2
Oxygen
3. Neutral
–CH –CH –O–
2 2
Fig. 10.3 A schematic representation of interaction of various nanoparticles with a PEO chain
and the LiSO 3 CF 3 ions: (1) Al 2 O 3 acidic, (2) Al 2 O 3 basic, and (3) Al 2 O 3 neutral.
Adapted with permission from Croce F, Persi L, Scrosati B, Serraino-Fiory F, Plichta E,
Hendrickson M. Role of the ceramic fillers in enhancing the transport properties of composite
polymer electrolytes. Electrochim Acta 2001;46(16):2457–61. Copyright 2001 Elsevier.
Simple oxide Ex situ • Polymer
Metal Polymer
• Salt
oxide Processing composite
Mixed oxide In situ • Additives electrolyte
Polymer
Filler electrolyte
Fig. 10.4 A stepwise representation of a PCE prepared with metal oxide-based fillers.
respectively, depending on the degree of crystallinity [38]. These materials include
three types, (i) sulfides, (ii) oxides, and (iii) phosphates.
Thio-LISICON is a lithium-ion-conducting sulfide crystal among other sulfides,
such as Li 2 S-P 2 S 5 , Li 2 S-Ga 2 S 3 -GeS 2 , and Li 2 S-Sb 2 S 3 -GeS 2 . Crystalline
Li 3.25 Ge 0.25 P 0.75 S 4 has conductivity as high as those of Li 2 S-P 2 S 5 glass-ceramics.
Perovskite, LLTO ((La,Li)TiO 3 ), is an oxide-based lithium-ion conductor. The La/
Li ratio determines the structure and ion conductivity. The difference in conductivity