Page 315 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 315
284 Polymer-based Nanocomposites for Energy and Environmental Applications
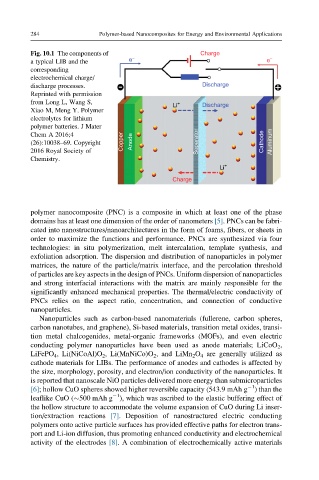
Fig. 10.1 The components of − Charge
a typical LIB and the e e −
corresponding
electrochemical charge/
discharge processes. Discharge
Reprinted with permission
from Long L, Wang S, Li + Discharge
Xiao M, Meng Y. Polymer
electrolytes for lithium
polymer batteries. J Mater
Chem A 2016;4
(26):10038–69. Copyright Copper Anode Separator Cathode Aluminum
2016 Royal Society of
Chemistry.
Li +
Charge
polymer nanocomposite (PNC) is a composite in which at least one of the phase
domains has at least one dimension of the order of nanometers [5]. PNCs can be fabri-
cated into nanostructures/nanoarchitectures in the form of foams, fibers, or sheets in
order to maximize the functions and performance. PNCs are synthesized via four
technologies: in situ polymerization, melt intercalation, template synthesis, and
exfoliation adsorption. The dispersion and distribution of nanoparticles in polymer
matrices, the nature of the particle/matrix interface, and the percolation threshold
of particles are key aspects in the design of PNCs. Uniform dispersion of nanoparticles
and strong interfacial interactions with the matrix are mainly responsible for the
significantly enhanced mechanical properties. The thermal/electric conductivity of
PNCs relies on the aspect ratio, concentration, and connection of conductive
nanoparticles.
Nanoparticles such as carbon-based nanomaterials (fullerene, carbon spheres,
carbon nanotubes, and graphene), Si-based materials, transition metal oxides, transi-
tion metal chalcogenides, metal-organic frameworks (MOFs), and even electric
conducting polymer nanoparticles have been used as anode materials; LiCoO 2 ,
LiFePO 4 , Li(NiCoAl)O 2 , Li(MnNiCo)O 2 , and LiMn 2 O 4 are generally utilized as
cathode materials for LIBs. The performance of anodes and cathodes is affected by
the size, morphology, porosity, and electron/ion conductivity of the nanoparticles. It
is reported that nanoscale NiO particles delivered more energy than submicroparticles
1
[6]; hollow CuO spheres showed higher reversible capacity (543.9 mAh g ) than the
1
leaflike CuO ( 500 mAh g ), which was ascribed to the elastic buffering effect of
the hollow structure to accommodate the volume expansion of CuO during Li inser-
tion/extraction reactions [7]. Deposition of nanostructured electric conducting
polymers onto active particle surfaces has provided effective paths for electron trans-
port and Li-ion diffusion, thus promoting enhanced conductivity and electrochemical
activity of the electrodes [8]. A combination of electrochemically active materials