Page 324 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 324
Polymer nanocomposites for lithium battery applications 293
interfacial cohesion while the material expands are needed in order to prolong the
active life of the Si particles.
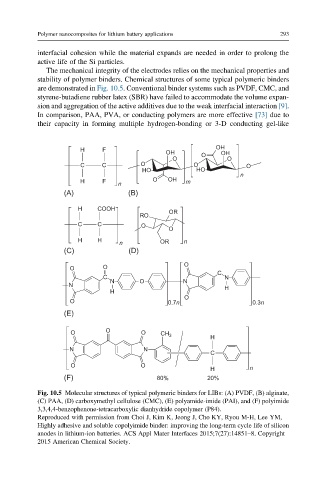
The mechanical integrity of the electrodes relies on the mechanical properties and
stability of polymer binders. Chemical structures of some typical polymeric binders
are demonstrated in Fig. 10.5. Conventional binder systems such as PVDF, CMC, and
styrene-butadiene rubber latex (SBR) have failed to accommodate the volume expan-
sion and aggregation of the active additives due to the weak interfacial interaction [9].
In comparison, PAA, PVA, or conducting polymers are more effective [73] due to
their capacity in forming multiple hydrogen-bonding or 3-D conducting gel-like
H F OH
OH O OH
O O
C C O O O
HO HO
n
H F n O OH m
(A) (B)
H COOH
RO OR
C C O
O
H H n OR n
(C) (D)
O
O O
C
C N
N O N
N H
H
O
O 0.7n 0.3n
(E)
O O O CH 3 H
N N
C
O O
H n
(F) 80% 20%
Fig. 10.5 Molecular structures of typical polymeric binders for LIBs: (A) PVDF, (B) alginate,
(C) PAA, (D) carboxymethyl cellulose (CMC), (E) polyamide-imide (PAI), and (F) polyimide
3,3,4,4-benzophenone-tetracarboxylic dianhydride copolymer (P84).
Reproduced with permission from Choi J, Kim K, Jeong J, Cho KY, Ryou M-H, Lee YM,
Highly adhesive and soluble copolyimide binder: improving the long-term cycle life of silicon
anodes in lithium-ion batteries. ACS Appl Mater Interfaces 2015;7(27):14851–8. Copyright
2015 American Chemical Society.