Page 335 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 335
Polymer nanocomposites for lithium battery applications 303
cathode lead to the formation of different polysulfides Li 2 S x (2<x 6). These
intermediate species are highly soluble in electrolytes based on glyme and dioxolane
solvents, which is responsible for the so-called polysulfide shuttle effect
(Fig. 10.11B). The polysulfides shuttle between the anode and cathode during the cell
cycling and react with both. Consequences are unwanted side reactions, deposition of
reactive species on the electrodes with significant increasing impedance of the cell,
sulfur depletion at the cathode, and lithium passivation and dendrite formation at
the anode. Therefore, research on polymer electrolytes and functional separators
has been really extensive in order to overcome these fundamental issues.
In addition, many advantages are foreseen in a smart design of the sulfur cathode by
engineering sulfur-polymer composite materials for improving the electronic conduc-
tivity, reducing the shuttle effect, and controlling sulfur structural and morphological
changes. To this aim, conductive polymers (e.g., polypyrrole, PANI, PEDOT, and
PAN) have been tested [104,105]. A striking example of an innovative design was
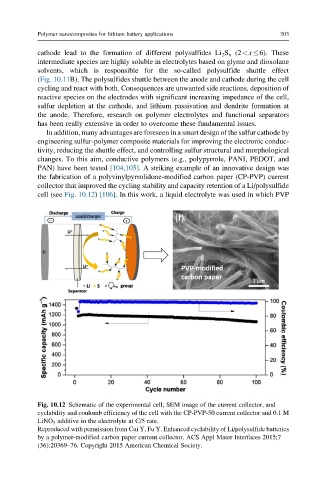
the fabrication of a polyvinylpyrrolidone-modified carbon paper (CP-PVP) current
collector that improved the cycling stability and capacity retention of a Li/polysulfide
cell (see Fig. 10.12) [106]. In this work, a liquid electrolyte was used in which PVP
Fig. 10.12 Schematic of the experimental cell, SEM image of the current collector, and
cyclability and coulomb efficiency of the cell with the CP-PVP-50 current collector and 0.1 M
LiNO 3 additive in the electrolyte at C/5 rate.
Reproduced with permission from Cui Y, Fu Y. Enhanced cyclability of Li/polysulfide batteries
by a polymer-modified carbon paper current collector. ACS Appl Mater Interfaces 2015;7
(36):20369–76. Copyright 2015 American Chemical Society.