Page 331 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 331
Polymer nanocomposites for lithium battery applications 299
C
CNS-700
CNS-850
CNS-1000
Normalized intensity (a.u.) O P
N
S
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2.2 2.4
Energy (keV)
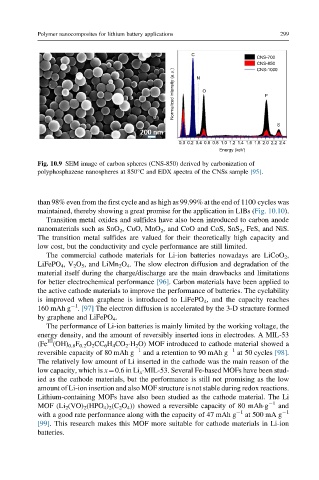
Fig. 10.9 SEM image of carbon spheres (CNS-850) derived by carbonization of
polyphosphazene nanospheres at 850°C and EDX spectra of the CNSs sample [95].
than 98% even from the first cycle and as high as 99.99% at the end of 1100 cycles was
maintained, thereby showing a great promise for the application in LIBs (Fig. 10.10).
Transition metal oxides and sulfides have also been introduced to carbon anode
nanomaterials such as SnO 2 , CuO, MnO 2 , and CoO and CoS, SnS 2 , FeS, and NiS.
The transition metal sulfides are valued for their theoretically high capacity and
low cost, but the conductivity and cycle performance are still limited.
The commercial cathode materials for Li-ion batteries nowadays are LiCoO 2 ,
LiFePO 4 ,V 2 O 5 , and LiMn 2 O 4 . The slow electron diffusion and degradation of the
material itself during the charge/discharge are the main drawbacks and limitations
for better electrochemical performance [96]. Carbon materials have been applied to
the active cathode materials to improve the performance of batteries. The cyclability
is improved when graphene is introduced to LiFePO 4 , and the capacity reaches
1
160 mAh g . [97] The electron diffusion is accelerated by the 3-D structure formed
by graphene and LiFePO 4 .
The performance of Li-ion batteries is mainly limited by the working voltage, the
energy density, and the amount of reversibly inserted ions in electrodes. A MIL-53
III
(Fe (OH) 0.8 F 0.2 O 2 CC 6 H 4 CO 2 H 2 O) MOF introduced to cathode material showed a
reversible capacity of 80 mAh g 1 and a retention to 90 mAh g 1 at 50 cycles [98].
The relatively low amount of Li inserted in the cathode was the main reason of the
low capacity, which is x¼0.6 in Li x MIL-53. Several Fe-based MOFs have been stud-
ied as the cathode materials, but the performance is still not promising as the low
amount of Li-ion insertion and also MOF structure is not stable during redox reactions.
Lithium-containing MOFs have also been studied as the cathode material. The Li
MOF (Li 2 (VO) 2 (HPO 4 ) 2 (C 2 O 4 )) showed a reversible capacity of 80 mAh g 1 and
with a good rate performance along with the capacity of 47 mAh g 1 at 500 mA g 1
[99]. This research makes this MOF more suitable for cathode materials in Li-ion
batteries.