Page 334 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 334
302 Polymer-based Nanocomposites for Energy and Environmental Applications
for the development of components for Li-air batteries. The same strategy has been
recently applied to check the stability of PMS in order to design an all-solid-state
Li-O 2 cell, which used a stable RM@GPE (redox mediator decorated GPE) engineered
to improve interfaces for lowering the overpotential (the difference between the charge
and discharge potential) and enhancing the efficiency. In more details, the GPE was a
nanocomposite of PP/PMS/TiO 2 membrane prepared through phase inversion with PP
as supporter and nano-TiO 2 as filler [86]. Concerns still remain about the stability of the
Limetalanodetowardtheredoxmediator(LiI)andtheeffectoflithiumcorrosiononthe
polymer nanocomposite electrolyte.
Material selection together with a thorough understanding of the reaction mecha-
nisms should be addressed before the Li-air technology could become a reality.
Without any doubts, the electrolyte for this system requires a careful design; oxygen
solubility and transport are critical. So far, a sort of Edisonian approach has been used
in order to determine the materials/designs for Li-air batteries. This kind of method is
inaccurate and time-consuming. Establishing criteria for electrolyte selection would
be essential [103]. In the field of polymer nanocomposite electrolytes, there is still
much more uncertainties and lack of fundamental studies on the oxygen transport
and mass transport in these materials.
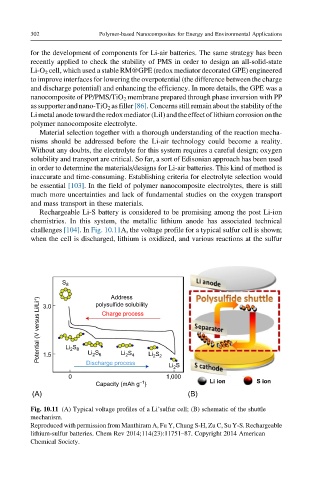
Rechargeable Li-S battery is considered to be promising among the post Li-ion
chemistries. In this system, the metallic lithium anode has associated technical
challenges [104].In Fig. 10.11A, the voltage profile for a typical sulfur cell is shown;
when the cell is discharged, lithium is oxidized, and various reactions at the sulfur
S 8 polysulfide solubility
Address
Potential (V versus Li/Li + ) Li S Li S Charge process
3.0
2 8
1.5
2 6
Li 2 S 4
2 2
Li S
2
0 Discharge process Li S 1,000
−1
Capacity (mAh g ) Li ion S ion
(A) (B)
Fig. 10.11 (A) Typical voltage profiles of a Li’sulfur cell; (B) schematic of the shuttle
mechanism.
Reproduced with permission from Manthiram A, Fu Y, Chung S-H, Zu C, Su Y-S. Rechargeable
lithium-sulfur batteries. Chem Rev 2014;114(23):11751–87. Copyright 2014 American
Chemical Society.