Page 367 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 367
334 Polymer-based Nanocomposites for Energy and Environmental Applications
12.1.1.1 Sodium ion batteries
The growing demand for green energy opened doors for development and research for
a large number of new technologies. As mentioned before, LIB are dominant in many
sectors, especially in consumer electronics, and their usage will increase in the follow-
ing years [5]. Although nowadays they are the most commonly used rechargeable
batteries, their capacity is still limited for the new demands. On the other hand, big
issue for LIB is the scarce lithium sources on the Earth [6]. Lithium as an element
can be extensively found in the Earth’s crust, but it is not an abundant one—just in
order only 20 ppm [7]. In addition, lithium sources are placed in tough regions, which
makes the access to them difficult. All these conditions affect the production cost of
LIB. On the contrary, sodium sources can be described as unlimited on Earth’s crust
(23,600 ppm [7]) and also in oceans [6,8]. In spite of its abundance, sodium is the
alkali metal with the smallest ion radius and atomic weight after lithium. Furthermore,
both technologies have similar working principle and production methodology [9].
Even though the lower cost of Na-ion technology is principally due to the abundant
sources of Na in the world, it also uses less expensive Na-salts as electrolytes, and does
not require usage of copper as anode current collector, which may reduce the total
material cost for about 11% [10]. All these facts make Na ion a promising chemistry
to replace the already commercialized Li-ion technology. However, Na-ion techno-
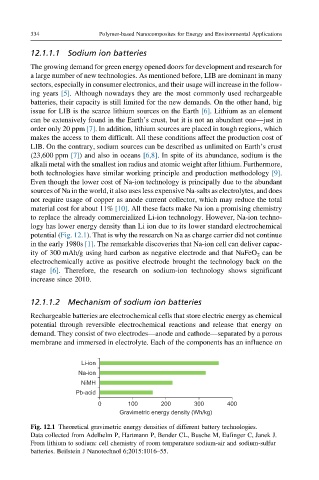
logy has lower energy density than Li ion due to its lower standard electrochemical
potential (Fig. 12.1). That is why the research on Na as charge carrier did not continue
in the early 1980s [1]. The remarkable discoveries that Na-ion cell can deliver capac-
ity of 300 mAh/g using hard carbon as negative electrode and that NaFeO 2 can be
electrochemically active as positive electrode brought the technology back on the
stage [6]. Therefore, the research on sodium-ion technology shows significant
increase since 2010.
12.1.1.2 Mechanism of sodium ion batteries
Rechargeable batteries are electrochemical cells that store electric energy as chemical
potential through reversible electrochemical reactions and release that energy on
demand. They consist of two electrodes—anode and cathode—separated by a porous
membrane and immersed in electrolyte. Each of the components has an influence on
Li-ion
Na-ion
NiMH
Pb-acid
0 100 200 300 400
Gravimetric energy density (Wh/kg)
Fig. 12.1 Theoretical gravimetric energy densities of different battery technologies.
Data collected from Adelhelm P, Hartmann P, Bender CL, Busche M, Eufinger C, Janek J.
From lithium to sodium: cell chemistry of room temperature sodium-air and sodium-sulfur
batteries. Beilstein J Nanotechnol 6;2015:1016–55.