Page 430 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 430
Polymer nanocomposites for dye-sensitized solar cells 387
1.00 1.00 1.00 20.0 nm
10.0 nm
0.75 0.75 0.75
0.0 nm
0.50 0.50 0.50
0.25 0.25 0.25
0 0 0
0 0.25 0.50 0.75 1.00 0 0.25 0.50 0.75 1.00 0 0.25 0.50 0.75 1.00
mm mm mm
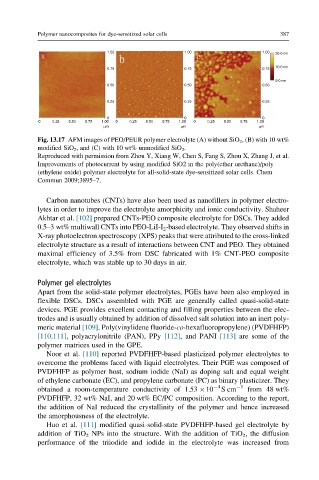
Fig. 13.17 AFM images of PEO/PEUR polymer electrolyte (A) without SiO 2 , (B) with 10 wt%
modified SiO 2 , and (C) with 10 wt% unmodified SiO 2 .
Reproduced with permission from Zhou Y, Xiang W, Chen S, Fang S, Zhou X, Zhang J, et al.
Improvements of photocurrent by using modified SiO2 in the poly(ether urethane)/poly
(ethylene oxide) polymer electrolyte for all-solid-state dye-sensitized solar cells. Chem
Commun 2009;3895–7.
Carbon nanotubes (CNTs) have also been used as nanofillers in polymer electro-
lytes in order to improve the electrolyte amorphicity and ionic conductivity. Shaheer
Akhtar et al. [102] prepared CNTs-PEO composite electrolyte for DSCs. They added
0.5–3 wt% multiwall CNTs into PEO-LiI-I 2 -based electrolyte. They observed shifts in
X-ray photoelectron spectroscopy (XPS) peaks that were attributed to the cross-linked
electrolyte structure as a result of interactions between CNT and PEO. They obtained
maximal efficiency of 3.5% from DSC fabricated with 1% CNT-PEO composite
electrolyte, which was stable up to 30 days in air.
Polymer gel electrolytes
Apart from the solid-state polymer electrolytes, PGEs have been also employed in
flexible DSCs. DSCs assembled with PGE are generally called quasi-solid-state
devices. PGE provides excellent contacting and filling properties between the elec-
trodes and is usually obtained by addition of dissolved salt solution into an inert poly-
meric material [109]. Poly(vinylidene fluoride-co-hexafluoropropylene) (PVDFHFP)
[110,111], polyacrylonitrile (PAN), PPy [112], and PANI [113] are some of the
polymer matrices used in the GPE.
Noor et al. [110] reported PVDFHFP-based plasticized polymer electrolytes to
overcome the problems faced with liquid electrolytes. Their PGE was composed of
PVDFHFP as polymer host, sodium iodide (NaI) as doping salt and equal weight
of ethylene carbonate (EC), and propylene carbonate (PC) as binary plasticizer. They
obtained a room-temperature conductivity of 1.53 10 4 Scm 1 from 48 wt%
PVDFHFP, 32 wt% NaI, and 20 wt% EC/PC composition. According to the report,
the addition of NaI reduced the crystallinity of the polymer and hence increased
the amorphousness of the electrolyte.
Huo et al. [111] modified quasi-solid-state PVDFHFP-based gel electrolyte by
addition of TiO 2 NPs into the structure. With the addition of TiO 2 , the diffusion
performance of the triiodide and iodide in the electrolyte was increased from