Page 173 - Pressure Swing Adsorption
P. 173
1:
: .li l
148 PRESSURE SWING ADSORPTION
EQUILIBRIUM THEORY 149
50
Where
THERMOCOUPLE
45
§ #2
40
w and
a:
C,
I-
-< 35
a: K=K; exp[AJI(J
w 0 -;)]
~ 0
" 30 is the temperature-dependent Henry's law coefficient of componen't 1. Com-
w
I-
I
t
25 j binmg these yields
20 0/3 KA AHA [ , ( Kl) AH. ) (4.67)
0 2400 4B00 7200 aT RT'[e/(1-e) +KA]' ! -, KA AHA - I
TIME {a)
+K.(tZ; -1)]
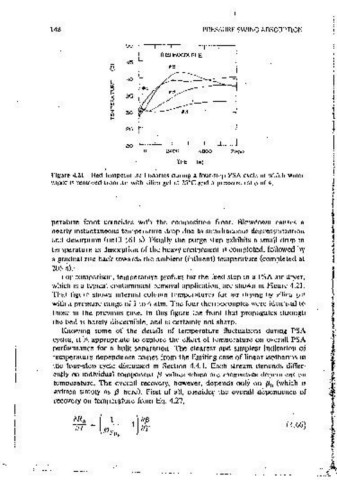
Figure 4.21 Bed tempcra1urc histories dunng a rour•step PSA cycle m which water
vapor 1s n::movcd from air with silica gel at 25°C an<l a pressure rallo of 4. i To cite a specific example, the parameters for separating oxygen from air
!
usmg zeolite SA at 45°C are: KA= 8.24, K = 4.51, AF/A= -6.0 kcal/mol,
8
AHB = -3.0 kcal/mo!, and,= 0.478, so of3A/aT= 0.00269 K-'. J8n/JT
2 30
= 0.00209 K-', and 0/3/oT = 0.00856 K-' "· At very high pressure rauos,
and desorotlon (until 161 s). Finally the purge step exhibits a small drop m I -0.00856 K- ': that IS, recovery would decrease by slightly less than l % if
perature front coincides with the composition front. Blowctown causes a only the second term m Eq. 4.66 1s important. and the limit 1s: i!R jaT =
8
nearly instantaneous temperature drop due to simuitaneous depressurizat1on
of 5, however, the first term m Ea. 4.66 is about -0.05, which would require
temoerature as ctesorptton of the heavy component 1s completed, followed by l the average temperature mcreased 1° C. At a more reasonable pressure ratio
a grndual nse back towards the ambient (influent) temoerature (completed at an average temperature increase of 20° C for recovery to decrease 1 %. At
205 s). iower pressure ratios, Eo. 4.66 predicts that the recovery would increase,
For comoarison, temoerature profiles for the feed step tn a PSA au dryer, rather than decrease, if the average temperature increased.
which ts a typical contammant removal application, are shown in Figure 4.21. Next, the flows mvolved m each step can be examined to see how
That ·figure shows mternal column temperatures for air drying by silica gei temperature fluctuations from step to step may affect .'the overall recovery.
with a pressure range of 1 to 4 atm. The four thermocouples were identical to That is, the temoerature dependence of each stream can be found from the
those in the previous case. In this figure the front that propagates through isotherm parameters. For the sake of discussion. auantities are identified
the bed is barely discernible, and is certainly not sharo. only by orders of magnitude, and the temperature of the high-pressure
Knowmg some ·of the details of temperature fluctuations during PSA product 1s taken as the base temperature. Relative to that, the temperature
cycles, 1t is appropnate to explore the effect of temperature on overall PSA reached at the end of oressunzation is practically thei same. The fact that
performance for a bulk separation. The clearest and simplest indication of pressurization begms at a lower temperature is less important. since eauili-
temperature cteoendence comes from the limiting case of linear isotherms in bration at the final temperature and pressure determines the quantity of gas
the four-step cycle discussed in Section 4.4.1. Each stream deoencts differ- admitted. The temperature encountered by the feed is higher (due' to the
ently on individual component /3 values which are themselves dependent on heat released by uptake). Finally, the temperature dunng blowdown (which
temoerature. The overall recovery, however, depends only on {¾ (which is does not affect recovery) and purge 1s lower due to :depressunzauon and
0
written simply as /3 here). First of all, consider the overall dependence of desorption. Hence, looking at each term m Ea. 4.26 reveals the effects of
recovery on temperature from Eq. 4.27, temperature shifts for mdividual steos:
0 - 0 - [Ml//3A)/ATl[ATlru] [-)[-]
( 4.66) [ A( l //3 A) I ti Tl[ ATIF l [-][+]
(4.68)