Page 282 - Pressure Swing Adsorption
P. 282
11
258 PRESSURE SWING ADSORPTION PSA PROCESSES 259
6.11 Efficiency of PSA Processes I >00000 r. ''I 'I .. " '1
I
The thermodynamic efficiency of any seoarat1on process (the First Law (a)
efficiency) can be defined simply as the ratio of the mmimum work of I i ,00000~
separation (the negative free energy of mixing) to the actual work required to ; f
dnve the separation process. Such ·efficiencies are generally less than 15% . '
: )00000~
and even iower than this for most PSA systems. The vaiues for two represen-
tative alf seoarat10n orocesses are given m Table 6.7. The nitrogen oroduc- ~ t
f
t1on orocess is markedly less efficient than oxygen production, reflectmg the I 0 f
irreversibility inherent m a kinetically based seoaratton. Although thermody-
namic efficiency provides a rational basis for comparing the efficiency of ~:;1°,Jt----------•·-· -~~~-"-7·-~----.;I
different PSA processes based on the same type of cycle, 11 1s much less
useful for comparing different types of separation process or even radically
different PSA cycles. Furthermore, the thermodynamic efficiency gives only :::·[ \:::__..---.!,' ·~"-, .----jj
an overall measure of oerformance and provides no mformation as to the
sources of efficiency. An exergy anaiys1s orovides far greater mstght. 0 )-9~ 10 l~"f,,~ ,.
The excrgy of a suhstance is the maximum useful work that can be i>RESSUIIE t8ARI
obtained by interaction with the environment. It is JO essence the free energy
I
relative to the normal environment as standard state. For a nonreactmg "f ' '
system in which potential and kinetic energies are ms1gnificant:
10 PU
Ex - (h - h ) - T (s - s ) (6.13)
0 0 0 20
The exergettc efficiency 1s then defined as: 18-1. PEI
moles product X Exproduct "
(6.14) ~
moles feed Ex feed 15
, Tr
' u
The feed exergy (Ex,,,.) mcludes the work of comoress1on while the w
u
~
oroduct exergy (Exproduc,) includes the energy of compression (or expans10n) ~ "
w I ,
to reduce the product to atrnosphenc pressure. For companng different PSA IO
w
processes, operated over different oressure ranges, a more useful definition is " f
~
~ '
the overall efficiency ( 1J) defined by: w
"
w
moiar exergy of product, corrected to l atm NPE
1J - net energy input ( 6.15) "
,,
where the net energy in out is ·the energy of feed comoress1on less the energy ,:
I
0
0 s " IO 10
Table 6.7. Thermodynamic (First Law) Effic1enc1es for PSA Air Separation Processes
PRESSURE lbor J
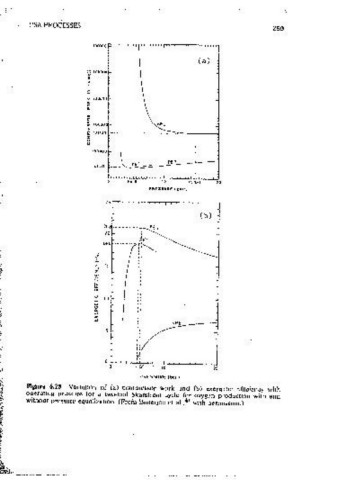
rrlnc1pul l"roces:. Enerjly SepilrullVe work Ellk1ency ')[. : uure 6.25 Var1ut!<m of (a) compressor work and (b) c"crgetlc cmciency with
1
Proces:; product (J /g mole product) (J/g mole product) (%) l;>eratmg pressure lor. a two-bed Skarstrom cycle for oxygen producuon with and
{!_ 411
"Lindox" 90% 02 4.8 X 10 4 3055 6.3 . ., without -Pressure equal1zat1on. (From Baner_1ce ct ai., with oernusslon.)
(Figure 6.8)
"Nilrotec" 3.2 X l0 4 660 2,1
(Figure 6.11 and 99% N 2
Table 6.l)