Page 277 - Pressure Swing Adsorption
P. 277
', ( .
254 PRESSURE SWING ADSORPTION PSA PROCESSES 255
modest ({3 = 0.5) and the initial moie fraction 1s .large (Yo= 0.5), the
!000
deviations from lirtearity, for both PA and f~ , are small. However, when the
3
r1-~- selectivity is high and the mJtial mole fraction of A is small, the curve for F, 1
--~ becomes essentially linear. but the curve for FA assumes a highlv nonlinear
I/min form. It is clear that, in this situation, by a sufficiently targe reduction m total
0.52 0
pressure almost all of component B can be desorbed with verv little desorn-
t.01 C, tion of A. A further deep blowdown or evacuation step then allows A to be
'~I nonlinearity 1s actually to enhance the degree of seoaration and concentra~
2.0
0.1 100 removed in concentrated form.
D
This analysis is for a linear eouilibrium system, but ·the effect of isotherm
\ 2:: 0 l t10n that can be achieved m this type of process. Since the 1soth·erm for the
more strongly adsorbed species will generally have the higher cutvature, even
~
less of this comoonent is desorbed dunng the imtial blowdown compared
with the eamvaJent linear equilihriurn svstem.
0.01 10 I. A process of this kind has rccentlv been developed as a means of
concentrating and removing the traces of tritium from the helium purge
stream of a lithium breeder reactor.-~~ To achieve a high concentration ratio
3
( ~ 10 ) requires a high select1v1ty ratio (as well as a high pressure ratio). and
for H (or tritium) this can be achieved only by qperating at cryogenic
2
yty temperatures with vacuum ctesorotion at a very low oressure. Laboratory data
~ showtng the feasibility of recovering hydrogen at greater than 90% punty and
"
with a similarity high fract10nal recovery from a stream containing traces of
0.001
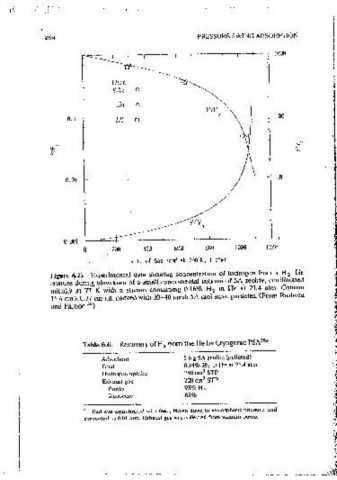
0 200 400 600 800 1000 1200 H 2 m He are summarized m Figure 6.22 and Table 6.6. The process
schematic 1s shown m Figure 6.23.
Vol. of Gas lcmJ at 298 K, l atml 37 3
The same principle was used by Yang and co-workers · tt in recent studies
Figure 6.22 · Expenmental data showing concentration of hydrogen from a /12-He of the possibility of removmg and concentratmg trace organics from air and
mixture dunng blowctown of a small experimental column of 5A zeolite, eqmllbratect 1' SO from flue gas. It 1s also utilized in the Air' Products fractional vacuum
2
initiaflv at 77 K with a stream containmg 0.16% H 2 in He at 21.4 atm. Column swing adsorption process (FVSA), whicl1 produces 90% oxygen together with
15.6 cTTlx0.77 cm 1.d. packed with 20-40 mesh SA mol sieve particles. (From Ruthven 98-99% nltrogen. 39 The cycle, which 1s essentially similar to that used m the
and Farooq. 36 ) -
hydrogen recovery process, mvolves four steps:
• Actsorot10n with feed air at 1.1 atm abs. with simurtaneous withctrawai of
oxygen product.
36
Table 6.6. Recovery of H from the He by Cryogemc PSA " Reverse flow blowdown with discharge of the blowdown gas (impure
2
Adsorbent 5.6 g 5A zeolite (pelleted) nitrogen) to waste.
Feed 0.J8%H 2 mHeat21.4atm Evacuat10n to 0.1 atm with collection and recompression of the nitrogen
Hydrogen uptake 240cmJ STP product.
Exhaust gas 220 cmJ STP Pressunzation with product oxygen.
Puntv 93%H 2
Recoverv 85%
With CaX zeolite as the adsorbent the seiecnv1ty (a ~ 10) 1s high enough
Bed was equilibrated with feed, blown down to atmospheric pressure, and that most of the oxygen ts elimmated m the blowdown step. About half the
cvacuated to 0.01 atm. Exhaust gas was collected from vacuum pump. adsorbed nitrogen can be recovered at 98-99% ounty. dunng the evacuation
step which run at about 0.1 atm abs. The schematic diagram together with
performance data are shown m Figure 6.24.