Page 57 - Pressure Swing Adsorption
P. 57
1 ·II i
32 PRESSURE SWING ADSORPTION FUNDAMENTALS OF ADSORPTION 33
where q~, CJ¼ arc the adsorbed phase concentrations of components A and Table 2.5. Published Equilibrium Data for Sorpllon of Atmosptlenc Gases
B, at the same spreading pressure, in the single-comoonent systems. To on Common Adsorbents 0
achieve this spreading pressure m the smgie-comoonent system the actual
Temp. range Pres~. range
pressure for the less strongly adsorbed component must be higher (in some (afm) Reference
Sorbent Sorbate (K)
cases much higher) than the total pressure m the binary system. The
development outlined here 1s for a binary system, but the extens,on to a 4A Zeolite Ar 200-300 0-0.8 Ruthven 16
200-300 0-0.7 Eagan!i
mult1comoonent system follows naturally. 111
200-300 0-1.0 Sprmger
It should be stressed that the assumption of ideal behavior defined by Ea. 1
306-363 0-·0.l Kumar ~
2.20 does not require a linear cquilibnum relattonship and does not preclude 4A Zeolitc 0 2 200-300 0-0.8 Ruthven 16
the possibility of interactions between the adsorbed molecules. The 1molica- 300-360 Henrv cons!. Haqw
t1on, however, 1s that any such interactions m the mixed adsorbed phase are 123-173 0-J.0 Eagan 11
77 0-Psat Stakebake~ 1
the same as m the smgle-comoonent systems. Such as assurnot10n is m fact
4A Zeolite 200-300 0-0.8 Ruthven 11,
Jess restnctive than it nught at first appear. However, it is difficult to tell a 20
300-360 Henry canst. Haq
a pnon whether or not this approximation 1s valid for any particular system. 305 0-J.0 Kumar 1 ';
To confirm the validity reouires at least limited expenmental data for the 195-223 0-1.0· Eagan 11
binary system. From the perspcci1vc of PSA modeling a more senous 5A Zeolite Ar 200-300 0-0.8 Ruthven 16
304-334 0-1.0 Kum1:1r ' 11
disadvantage of the ideal adsorbed solution theory (IAST) approach 1s t.hat 1t 22
203-297 0-4.5 Miller
provides the equilibrium relationship m 1mplic1t rather than explicit form. 21
195-348 0-J 1.0 Waka.~ugi
This makes it inconvement for direct mcoroorat10n mto a numencal sm10la- 5A Zeo\ite o, 200-300 0-0.8 Ruthven t h
t1on code, 300-350 Henrv const. Haqz"
300-394 0-1.0 Kumar ' 1 1
203-297 0-4.5 Miller 22
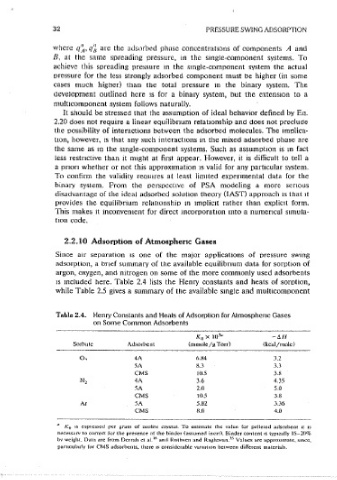
2.2. l O Adsorption of Atmospheric Gases 273-303 0-0.8 Sorral 24
144 0-2.1 Danner2.~
Since air separation ts one of the major applications of pressure swing 77 0-P,at Stakebake 21
adsorption, a bnef summary of the available equilibnum data for sorotion of 298 0-0.8 Huang;n
1
argon, oxygen, and nitrogen on some of the more commonly used adsorbents SA Zeolite 200-300 0-0.8 Ruthven "
2
Henry canst.
300-360
Haq ~
1s mclucted here. Table 2.4 lists the Henry constants and heats of sorotion,
300-421 0-1.0 Kumar 19
while Table 2.5 gives a summary of the available smgle and multicomponent 25
144 U-1.0 Danner
1
200-300 0-1.0 Spnnger a
2
195,295 0-30 Lederman "'
Table 2.4. Henry Constants and Heats of Adsorption for Atmospheric Gases 203-297 0-4.5 Miller 22
on Some Common Adsorbents - 278-303 0-5 S{mal 24
76 P,,., Kidnav lk
7
K 0 X 10 " -~II 77-348 0-17.5 Wakasugi 21
Sorhatc Adsorbent (mmole/gTorr) (kcUljmole) 274-348 fl-4.2 Verelst ''
2
5A Zeolite 283-323 1.0 van der Vli!>t 1 1
0, 4A 6.R4 3.2
144 1.0 Danner'-~
SA R.:\ 3.3 2<J~,304 1.0 Kumar ''
1
CMS 10.5 3.8 299,320 1.0-4JJ Verels1 29
N, 4A 3.6 4.35 24
278-303 l.7-4.4 Sonal
SA 2.0 5.0 298 0.2-4.0 Miller 22
CMS 10.5 3.8 25
144 0-1.8 Danner
Ar SA 5.82 3.36 144 0-2.9 Dorfman 32
CMS 8.0 4.0 3
172-273 0-2.l Nolan l
5A Zeolik 144 0-2.9 Dorfman 32
" K 0 1s exp1-essed per gram of zeolik ervslal. To c.s11mate the value for pelleted adsorbem 11 1s 3
78-273 0-2.1 Nolan.1
necessarv to correct for the presence ot 1he bimlcr <assumed inert). Binder content 1s typ1Ci1lly 15-20% 25
1 144 0-i.2 Danner
bv weight. D,Ha are from Di.::rrnh ct al. 1> and Ruthven and Raght1viln.~~ Vt1lues are approximate, smce,
parucularly for CMS adsorbents, there 1s considerable vana11on bc1ween different materials. (Continued)