Page 63 - Pressure Swing Adsorption
P. 63
38
PRESSURE SWING ADSORPTION FUNDAMENTALS OF ADSORPTION 39
12 r---------------------.
A strong concentrat1on dependence of the rrncrop()rc diffu.r;,1V1tv 1~ com-
(a) monly observed. and m many cases this can be accounted for s1mpiv hy
0
10 considermg the effect of svstem nonlineantv. The true dnving force for anv
diffusive process is the gradient of chemical ootentiai, rather than the
• gradient of concentration, as assumed in the Fickian formulation:
6
0
dµ,
1 = -BqtlZ (2.29)
where the chem1ca1 ootenwd 1s related to tile act1v1tv by:
0
• µ. = µ,° + RT In a (2.30)
0
L----o For an ideal vapor phase the activity 1s essentially equal to the nartJai
Pressure; so Eqs. 2.29 and 2.30 reduce to:
0 (2 31)
C (Wt%)
6 ' . '
2 3 4 5 6
(b) '
5
(c)
> • 20
323 K
. ..J
4 4 .
371 K
. IL'';. ............. ! .. /A 371. K
. .
'v ... . ........
• . • • •• • -1 • 358.9 K
- 3_ • 10 •••
"g
•
>< ♦• .. B
•
" C ~.' T
0
---- ... 0 ~ 6 • ••••• ..J 323 K
~
• •••
•• 273 K ~ • • ••••• • ••
2 Q
358,9 K X
.. N .:: 4
. 00
•
•
2
••• • • ••••••2T3°K
• •••••
1 ";;;---:!-'---:!------,!-'---,~·--.,L---c-! 7
o.o 2 4 6 8 10 12
'
'
Co nee ntrat1on; Mol.iCavity 7 B 9
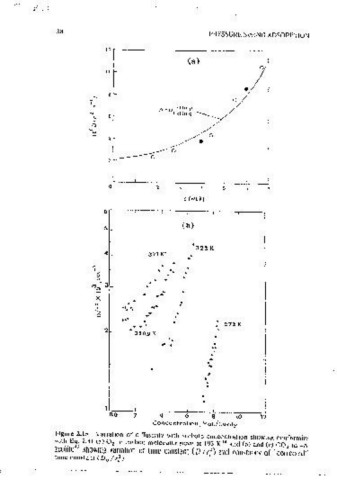
Fi_gure 2.15 Variation of ditfus1v1tv with sorhatc conccnLntt1on showing conformity Concenlrot1on; Molecules/Covily
14
wllh Eq. :!.31 (a) 0 2 m carbon molecular sieve at 193 K and (b) and (c) CO~ m 4A
1
zeolite" showmg vanauon of lime constant (D / r, ) and constancv of "corrected" Figure 2.15 (Continued).
ume constant (D /r}). · ·
0