Page 66 - Pressure Swing Adsorption
P. 66
:1.1
40 PRESSURE SWING ADSORPTION FUNDAMENTALS OF ADSORPTION 41
where D is the Fickian diffus1v1ty, defined m the usual way by: 14r--------------------,
J - _ 0 da (2.32) /0
dz 12
ln the limit of a linear system (Henry's Law) d In p / d In tJ -"' 1.0 and the ;,A
0/
Fickian diffusivity becomes mctependent of concentrat1on. For most micro-
10 SA
porous adsorbents, however, the isotherm 1s of type I form; so. Eo. 2.31
predicts an mcreasmg trend of diffus1v1tv with concentration. In particular, ., MSC-SA .
for the Langmmr isotherm (Eo. 2.3):
0 8
E / O
q/q = ~· /2.33) 0
- ' 6
0
u
from which it may be seen that, m the saturation region, the concentration -"
dependence 1s very strong. Although there is no sound theoretical reason to
expect the corrected diffus1v1ty ( D ) to be tndependent of concentration, this
0
pattern of behavior has been observed experimentally for several sorbates on
2
10
10· 1
"" c. (Ai
~ 3
\CT (c)
":!'
' Figure 2.16 (Contuwed).
0
10·t
both small-pore zeolite and carbon molecular s1cvtt adsorbents (see Figure
10·
H• H._ 0.."'-.CON o-1,. 4"1.,. 2. 15).
,
2 ' 4 5 Micropore diffusion 1s an activated process; so, m contras[ to molecular or
Molecular Diameter (A.) Knudsen diffusiv1t1es, the temperature dependence 1s strong and generally
(a) follows the Arrhemus form:
(2.34)
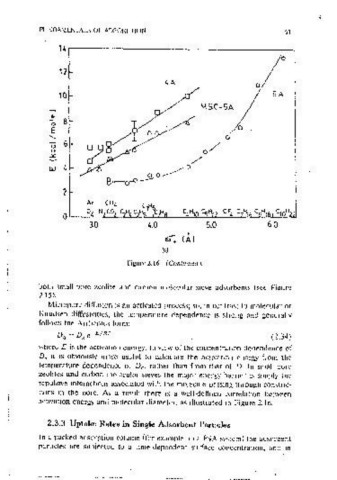
-;;; 30 ~, where Eis the act1vat1on energy. In view of the concentration dependence of
1\ 20 D; It IS obviously more useful to calcuiate the act1vat1on energy from the
' 0 ~
~ 10 ! temperature dependence of D , rather than from that of D. Jn small-oore
0
H zeolites and carbon molecular sieves the maJor energy barner is simply the
"' 0 0 2 3 4 5 repulsive mteractions associated with the molecule oassmg through constric-
Molecular Diameter (Al tions m the pore. As a result there 1s a well-defined correlation between
(b) activation energy and molecular diameter. as illustra,ted 1n Figure 2.16.
Figure 2.16 Correlation of.diffusivity and diffus10nal activation energy with molecu-
lar diameter for several sorbates m 4A and 5A zeolites and carbon molecular sieves. 2.3.3 Uptake Rates in Single Adsorbent Particles
ta) Diffusional time constants for differeni molecuiar sieve carbons; (b) and (c)
diffusional activatton energies: for vanous molecular sieve carbons and 4A and SA In a packed adsorpt10n column (for example, ma PSA system) the adsorbent
1
zeolites. (From SchrOter and Jiintgen 36 and Ruthven, with perm1ss1011.) particles are sub_jected to a ume-dependent surface concentration, and in