Page 71 - Pressure Swing Adsorption
P. 71
i. I
46 PRESSURE SWING ADSORPTION FUNDAMENTALS OF ADSORPTION 47
10-2~-----------,
10- 3
10·6
-
<11
N
E
u
N : E:6.5 kcal/mole o'
2
10· 1
10-5'--...!---'----'----'--'
10 3.2 3.4 3.6
10; IT (K"')
(a)
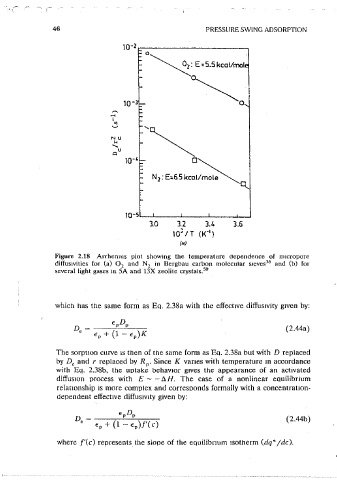
Figure 2.18 Arrhenms piot showing t11e temperature dependence of m1cropore
diffus1vities for (a) 0 2 and N 2 in Bergbau carbon molecular s1eves 35 and (b) for
several light gases in 5A and 13X zeolite crystals. 50 3
10 /T (K"l
(b)
Figure 2.18 (Contmued).
which has the same form as Eq. 2.38a with the effective diffus1v1ty given by:
2.3.8 Heat Transfer Control
(2.44a)
Since adsorption or ctesorot1on 1s generally associateQ with a s1gnificam heat
effect (exothermic for adsorotion), sorption/desorot10n rates may be influ-
The sorptlon curve 1s then of the same form as Eu. 2.38a but with D replaced enced or even controlled by the rate of heat dissipation. Such effects have
by D~ and r replaced by Rr. Since K vanes with temperature m accordance heen mvest1gated both theoretically and expcnmentally. 45 4
· ri In the limitmg
with Eq. 2.38b, the uptake behavior gives the appearance of an activated
situation in which all mass transfer processes are rapid, the sorpt1on rate 1s
diffusion process with £ ~ -6.H. The case of a nonlinear eauilibrium
controlled entirely by the rate of heat dissmation, and the sorpt1on/desoro-
relationship 1s more complex and corresoonds formally with a concentrat1on-
tion curve assumes a very s1mpie form:
cteoendent effective difl'us1v1ty given by:
m. B I ha t \
1
m: ~ - l + B exp l - C, (1 + B) j (2.45)
(2.44b)
The exoenmental adsorot1on/desorpt1on curves for carbon dioxide m 5A
zeolite crystals, presented m Figure 2.19, conform to this s1moie model. As
where f'(c) represents the slope of the equilibnum isotherm (dq* /de). with the diffusion or surface resistance mass transfer models, the approach to