Page 75 - Pressure Swing Adsorption
P. 75
50 PRESSURE SWING ADSORPTION FUNDAMENTALS OF ADSORPTION 51
100 ~------------------ 10
A
4
10- '-------'--------'------__,
0 100 200 300
r (sl 0. I
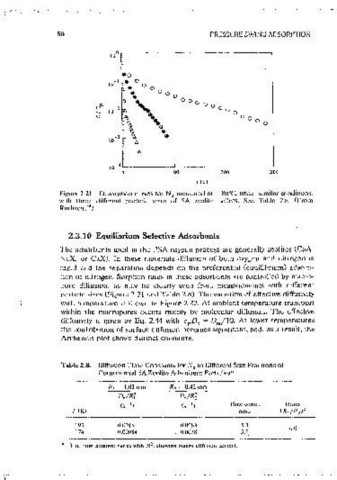
Figure 2.21 DcsorptlOn curves for N, measured at ~ 80"C, under similar condition~,
with three different particle sizes Of SA zcolite pellets. See Table 2.8. (From
Ruthven. 54 )
2.3. 10 Equilibrium Selective Adsorbents
9.01
The adsorbents used m the PSA oxygen process are generally zeolites (CaA,
3.4 3.8 4.2 5.0 5.4 5.8
NaX, or CaX). In these matenals diffusion of both oxygen and nitrogen 1s
raoid and the senaration depends on the preferential (eouilibnum) adsorp-
tion of nitrogen. Sorpt1on rates m these adsorbents are controlled by macro~
Figure 2.22 Arrhenws plot showing varrntton of effective (macropore) diffus1v1iv with
norc diffusion, as mav be clearly seen from measurements with different temperature for 0 and N m pelleted SA zco!itc. (From Ruthvcn.-~ )
4
particle sizes (Figure 2.21 and Table 2.8). The vanation of effective ditfus1v1ty 2 2
with temnerature 1s shown m Figure 2.22. At ambient temperature transport
within the macropores occurs mainly by molecular diffusion. The effective
diffusivity 1s given by Eq. 2.44 with EPDP ~ D /10. At lower temperatures
111 2 .3 .11 Separation Factor and Selectivity
the contribut10n of surface diffus10n becomes significant, and, as a result, the
Arrhenms plot shows distmct curvature. In an equilibrium based separation the select1v1ty of the adsorbent 1s gov-
erned by the separation factor. defined in EC.l. 2.14. For a Langmuir system
this factor 1s equivalent to the ratio of the · Henr/s Law constants. so
Table 2.8. Diffusion Time Constants for N in Different Size Fractions of comoarison of the Henry constants (or the chromatographic retention vol-
2
Commercial SA Zeolile Adsorbent Particles" umes which arc directly related tn the Henry constants through Eq, 2.61)
provides a s1molc and convenient approach for preliminary screening of
R! = 1.03 mm R 2 = 0.42 mm
potential adsorbents.
De/Rf D.jR~
In a kinetically controlled separatmn process the situation 1s somewhat
(s -1) (s- l) Time const. Ratio
T(K) ra110 (R 1 /R~) 2 more complicated, smce the selectivity then depends nn both kinetic and
equilibrium effects. In a membrane type of process which operate~ under
193 0.0016 0.00S3 5.3 6.0 steady-state conditions (see Section 8.1 ), the seiiarat1on factor, at high
174 0.00064 ().()038 5.9 pressure ratios, approaches the permeahilitv ratio (Eq. 8.8) 1.e .. the product
of the ratio of diffus1vities and equilibrium constant~. The reduction in
The um.:: co11srnnt v11r1cs wllh R", showing macro diff11swn conirol.