Page 140 - Principles and Applications of NanoMEMS Physics
P. 140
128 Chapter 3
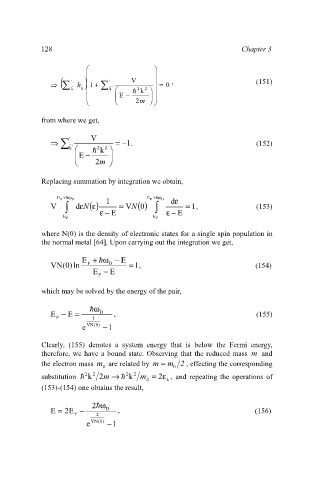
§ ¨ · ¸
( ' ¨ ' V ¸ , (151)
) 1 +
¦ G h G k ¨ ¦ k G ' 2 2 ¸ = 0
k
¨ § ¨ E − = k · ¸ ¸
¨ ¨ ¸ ¸
© © 2m ¹ ¹
from where we get,
' V
G ' ¦ = − 1. (152)
§ = k ·
k 2 2
¨ E − ¸
¨ ¸
© 2m ¹
Replacing summation by integration we obtain,
E F += ω D 1 E F += ω D dε
V ³ dεN () ε ε − = VN () 0 ³ ε − = 1, (153)
E E E E
F F
where N(0) is the density of electronic states for a single spin population in
the normal metal [64]. Upon carrying out the integration we get,
E + = ω − E
VN ) 0 ( ln F D = 1, (154)
E − E
F
which may be solved by the energy of the pair,
= ω
E − E = D . (155)
F 1
e VN ) 0 ( − 1
Clearly, (155) denotes a system energy that is below the Fermi energy,
therefore, we have a bound state. Observing that the reduced mass m and
the electron mass m are related by m = m 2 , effecting the corresponding
0 0
2
m
substitution = 2 k 2 2 → = 2 k m = 2ε , and repeating the operations of
0 k
(153)-(154) one obtains the result,
2 ω
=
E = 2 E − D . (156)
F 2
e VN ) 0 ( − 1