Page 191 - Principles of Catalyst Development
P. 191
CATALYST CHARACTERIZATION 179
CO METHANATION ON NICKEL CATAYLST
1.0
'I 340"C
a: _-0---0 -----0-
J:
~
I
CI
rn
w
....I 0.5
0
~
u.i
.....
e( 250"C
a: __ ----cr--~~-------,-o-
0
1000 1500 2000 2500
IMPELLER RPM
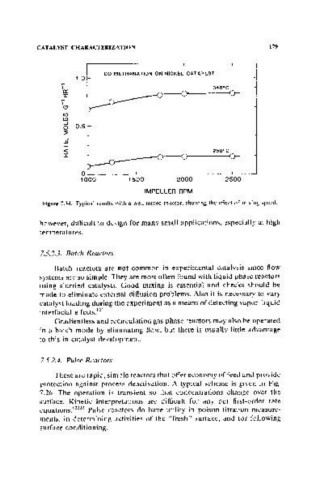
Figure 7.34. Typical results with a well·mixed reactor, showing the effect of mixing speed.
however, difficult to design for many small applications, especially at high
temperatures.
7.5.2.3. Batch Reactors
Batch reactors are not common in experimental catalysis since flow
systems are so simple. They are most often found with liquid phase reactors
using slurried catalysts. Good mixing is essential and c:hecks should be
made to eliminate external diffusion problems. Also it is necessary to vary
catalyst loading during the experiment as a means of detecting vapor-liquid
interfacial effects. (21)
Gradientless and recirculation gas phase reactors may also be operated
in a batch mode by eliminating flow, but there is usually little advantage
to this in catalyst development.
7.5.2.4. Pulse Reactors
These are rapid, simple reactors that offer economy of feed and provide
protection against process deactivation. A typical scheml~ is given in Fig.
7.26. The operation is transient so that concentrations change over the
surface. Kinetic interpretations are difficult for any but first-order rate
equations.(255) Pulse reactors do have utility in poison titration measure-
ments, in determining activities of the "fresh" surface, and for following
surface conditioning.