Page 209 - Principles of Catalyst Development
P. 209
198 CHAPTER 8
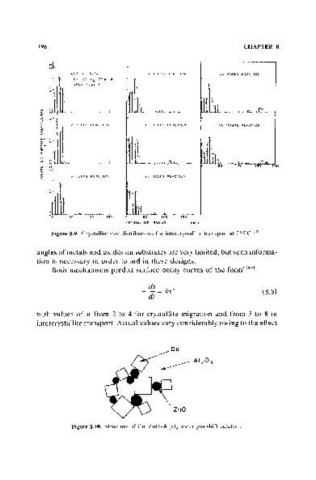
N
o
40't" N, $,02 10 HOURS REACTION 3S HOUAS REACTION
15'\ CO H2 CO=18
FAESH SAMPLE
o·
2 HOURS REACTION 15 HOuRS REACTION
!: ~ Il!11
ffi
~
~
i5
200
5 HOURS REA.CTION :2 5 HOURS REACT ION
50 100 15.0 50 100 15.0
CRVS"'fAllITE RADIUS I nm I
Figure 8.9. Crystallite size distributions for intercrystallite transport at 225°C. (267)
angles of metals and oxides on substrates are very limited, but such informa-
tion is necessary in order to aid in these designs.
Both mechanisms predict surface decay curves of the form (265)
ds
- - = ks" (8.5)
dt
with values of n from 2 to 4 for crystallite migration and from 5 to 8 in
intercrystallite transport. Actual values vary considerably owing to the effect
Cu
Figure 8.10. Structure of Cu-ZnO-AI 20, water gas shift catalyst.