Page 206 - Principles of Catalyst Development
P. 206
CATALYST DEACTIVATION 195
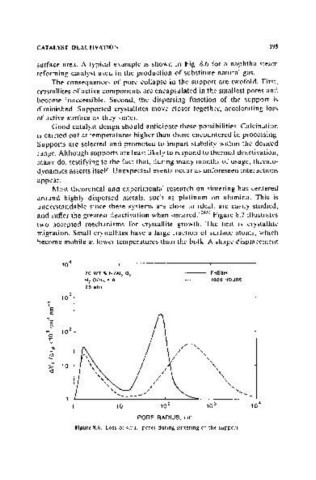
surface area. A typical example is shown in Fig. 8.6 for a naphtha steam
reforming catalyst used in the production of substitute natural gas.
The consequences of pore collapse in the support are twofold. First,
crystallites of active components are encapsulated in the smallest pores and
become inaccessible. Second, the dispersing function of the support is
diminished. Supported crystallites move closer together, accelerating loss
of active surface as they sinter.
Good catalyst design should anticipate these possibilities. Calcination
is carried out at temperatures higher than those encountered in processing.
Supports are selected and promoted to impart stability within the desired
range. Although supports are least likely to respond to thermal deactivation,
many do, testifying to the fact that, during many months of usage, thermo-
dynamics asserts itself. Unexpected events occur as unfort:seen interactions
appear.
Most theoretical and experimental research on sintering has centered
around highly dispersed metals, such as platinum on alumina. This is
understandable since these systems are close to ideal, are easily studied,
and suffer the greatest deactivation when sintered. (265) Figure 8.7 illustrates
two accepted mechanisms for crystallite growth. The Hrst is crystallite
migration. Small crystallites have a large fraction of surface atoms, which
become mobile at lower temperatures than the bulk. A shape displacement
104r---------~----------_.----------,_--.------~
70 WT" Ni/AI2 0 3 FRESH
H2 0/H 2 = 9 1000 HOURS
25 aIm
~
I
E
I::
"'E
u
III
o
,...
>< ",-,
... Q, "
<l I / / "
.....
"
"
;:: 10 "
<l " ,
" \
" " ' ........
10 10 2
PORE RADIUS, nm
Figure 8.6. Loss of small pores during sintering of the support.