Page 201 - Principles of Catalyst Development
P. 201
190 CHAPTER 8
REACTION I
__
ZONE ~ ~ ________ ~ __ --~
TOUT
POISON I
LU DEPOSITED I
a:
::J TIN
I-
04:
a:
LU TOUT
a.
:::!:
LU
I-
a:
0
I-
<..>
04:
LU TIN
a:
TOUT
BED DEPTH
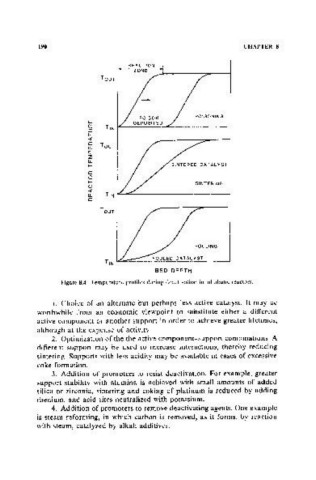
Figure 8.4. Temperature profiles during deactivation in adiabatic reactors.
1. Choice of an alternate but perhaps less active catalyst. It may be
worthwhile from an economic viewpoint to substitute either a different
active component or another support in order to achieve greater lifetimes,
although at the expense of activity.
2. Optimization of the the active component-support combinations. A
different support may be used to increase interactions, thereby reducing
sintering. Supports with less acidity may be available in cases of excessive
coke formation.
3. Addition of promoters to resist deactivation. For example, greater
support stability with alumina is achieved with small amounts of added
silica or zirconia, sintering and coking of platinum is reduced by adding
rhenium, and acid sites neutralized with potassium.
4. Addition of promoters to remove deactivating agents. One example
is steam reforming, in which carbon is removed, as it forms, by reaction
with steam, catalyzed by alkali additives.