Page 158 - Radiochemistry and nuclear chemistry
P. 158
Absorption of Nuclear Radiation 143
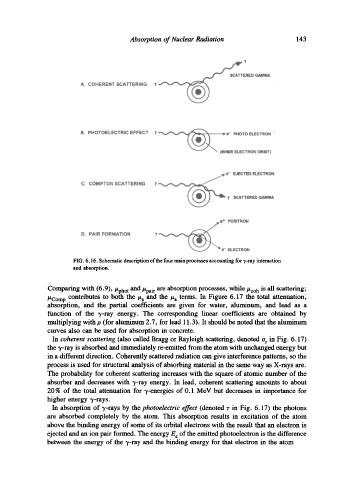
FIG. 6.16. Schematic description of the four main processes accounting for 7-ray interaction
and absorption.
Comparing with (6.9),/~phot and/~pair are absorption processes, while/~coh is all scattering;
/~Comp contributes to both the/~s and the/~a terms. In Figure 6.17 the total attenuation,
absorption, and the partial coefficients are given for water, aluminum, and lead as a
function of the 7-ray energy. The corresponding linear coefficients are obtained by
multiplying with p (for aluminum 2.7, for lead 11.3). It should be noted that the aluminum
curves also can be used for absorption in concrete.
In coherent scattering (also called Bragg or Rayleigh scattering, denoted a r in Fig. 6.17)
the 7-ray is absorbed and immediately re-emitted from the atom with unchanged energy but
in a different direction. Coherently scattered radiation can give interference patterns, so the
process is used for structural analysis of absorbing material in the same way as X-rays are.
The probability for coherent scattering increases with the square of atomic number of the
absorber and decreases with -y-ray energy. In lead, coherent scattering amounts to about
20% of the total attenuation for "r-energies of 0.1 MeV but decreases in importance for
higher energy "y-rays.
In absorption of 7-rays by the photoelectric effect (denoted r in Fig. 6.17) the photons
are absorbed completely by the atom. This absorption results in excitation of the atom
above the binding energy of some of its orbital electrons with the result that an electron is
ejected and an ion pair formed. The energy E e of the emitted photoelectron is the difference
between the energy of the "r-ray and the binding energy for that electron in the atom