Page 155 - Radiochemistry and nuclear chemistry
P. 155
140 Radiochemistry and Nuclear Chemistry
6.4.5. Absorption curves and scattering of f3-particles
An absorption curve for B-particles has a quite different shape than it has for a-particles
(of. Fig. 6.3). The continuous spectrum of energies in radioactive B-decay plus the
extensive wide angle scattering of the B-particles by the absorber atoms account for the fact
that range curves for B-particles continuously decrease. Even for a ~ of initially
mono-energetic electrons, the continuous removal of electrons from the beam path by wide
angle deflection results in a plot showing a continuous decrease in the numbers of electrons
with distance, with approximately 95 % of the original B-particles stopped in the first half
of the range. It is more common to speak of the absorber thickness necessary to stop 50 %
of the particles than to speak of the range itself. This half-thickness value is much easier
to ascertain experimentally than is an apparent range. It should be remembered that the
energy deposited at complete B-absorption is Eab s ~ Emax/3 (Ch. 4).
The absorption curve for B-particles formed in radioactive decay can be describe~ with
fair approximation by the relationship (6.7). This is due to the continuous energy spectrum
resulting in an exponential relationship for the range curve. In the Ema x range 0.7-3 MeV
the range in aluminum closely follows the relation (Feather's rule)
R(g AI em -2) = 0.543 Emax(MeV ) - 0.160 (6.18)
This is the range C 3 in Figure 6.4.
Figure 6.12 shows an empirical relationship between the maximum energy of B-particles
and the extrapolated range in aluminum. Compared to c~-radiation, B-radiation has a much
longer range. For example, the range of an a-particle of 5 MeV is 3.6 cm in air while that
of a B-particle of 5 MeV is over 17 m. A comparison of the range in air and water for
electrons and heavy particles is given in Table 6.2. Figure 6.13 is useful for a rapid
estimate of the absorber thickness n~xled to protect against B-particles.
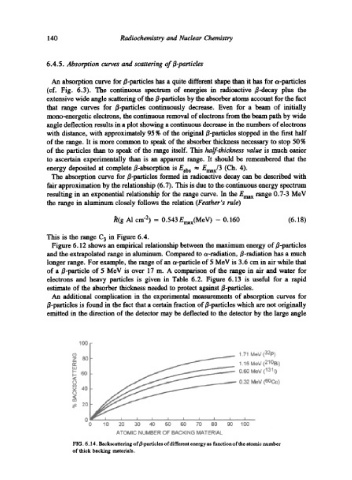
An additional complication in the experimental measurements of absorption curves for
B-particles is found in the fact that a certain fraction of B-particles which are not originally
emitted in the direction of the detector may be deflected to the detector by the large angle
FIG. 6.14. Backscattering of~particles of different energy as function of the atomic number
of thick backing materials.